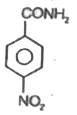
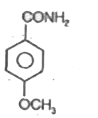
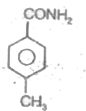
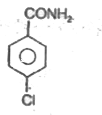
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The most reactive compound towards Hofmann bromamide degradation is
Text Solution
|
- RCONH2 is converted into RNH2 by means of Hofmann bromamide degradatio...
Text Solution
|
- Product of this Hofmann bromamide reaction is
Text Solution
|
- In the Hofmann-Bromamide rearrangement intermediate compounds are:
Text Solution
|
- RCONH(2) is converted into RNH(2) by means of Hofmann bromamide dehydr...
Text Solution
|
- Which amide will be most reactive for Hofmann- bromamide rearrangement...
Text Solution
|
- Which of the following statement(s) is/are incorrect in case of Hofman...
Text Solution
|
- Which of the following statement(s) is/are incorrect in case of Hofm...
Text Solution
|
- Write the reactions involved in the following: Hofmann bromamide degra...
Text Solution
|