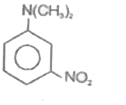
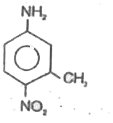
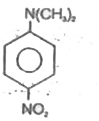
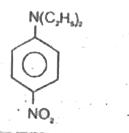
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 4-Nitro-N,N-dimethyl benzenamine is
Text Solution
|
- A student tried to synthesis 4-nitro-N, N-dimethylaniline from N, N-di...
Text Solution
|
- परिवर्तन करें --- बेंजीन से N, N डाइमेथिल एनीलिन
Text Solution
|
- N,N dimethyl acetamide is
Text Solution
|
- N,N - dimethyl anilinium acetate obtained from
Text Solution
|
- निम्न को उनकी बढ़ती क्षारकता के क्रम मे व्यवस्थित कीजिये। - p-टॉलूइडी...
Text Solution
|
- Draw the structural formula of the following compounds. (i) N-ethyl-N-...
Text Solution
|
- Benzaldehyde condenses with N, N - dimethyl aniline in the presence of...
Text Solution
|
- 4-Nitro-N,N-dimethyl benzenamine is
Text Solution
|