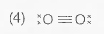A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-MOCK TEST 5-Example
- The correct order of ionic radii of the following species is
Text Solution
|
- Ammong the following, the element having highest ionization enthalpy a...
Text Solution
|
- Addition of an electron to a neutral gaseous atom alwayds leads to (i...
Text Solution
|
- Predict correct statement.
Text Solution
|
- In which of the following pairs, the ionisation energy of the first sp...
Text Solution
|
- The number of elements which can be accommodated in the present set-up...
Text Solution
|
- Correct order of ionisation enthalpy is
Text Solution
|
- Which of the following is correct for electron gain enthalpy (with -ve...
Text Solution
|
- An element with atomic number 113 has been discovered.It will belong t...
Text Solution
|
- In the general electronic configuration (n-2)^f(1-14) (n-1)^d(0-1) ns^...
Text Solution
|
- Which of the following is a metalloid?
Text Solution
|
- The X-X bond length is 1.5 Å and Y-Y bond length is 1.48 Å.If electron...
Text Solution
|
- The common valency shown by the elements of group 15 is/are
Text Solution
|
- Out of the following elements,which one is the most reactive chemicall...
Text Solution
|
- What is the covalency of Al in [AlCl(H2O)5]^(2+) ?
Text Solution
|
- Which of the following oxides is most basic in nature?
Text Solution
|
- Elements of which block generally show maximum variation in valency?
Text Solution
|
- In modern periodic table,the basic character of oxides
Text Solution
|
- Beryllium and aluminimum exhibit many properties which are similar ....
Text Solution
|
- Which of the following is the Lewis structure of O2 molecule?
Text Solution
|