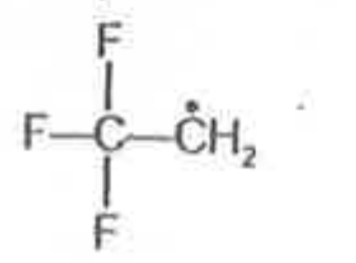
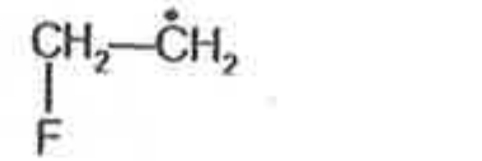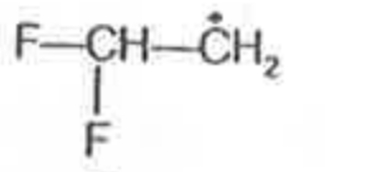A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-MOCK TEST 31-Example
- The shape of CH3^(+) and CH3^(-) is respectively
Text Solution
|
- Non-aromatic compound is
Text Solution
|
- Most stable radical among the following is
Text Solution
|
- In the given species, carbanion is sp^3 hybridised?
Text Solution
|
- Which of the following carbocation is most stable?
Text Solution
|
- The correct stability order of the given carbanions is
Text Solution
|
- Aromaticity order for the following aromatic compound will be
Text Solution
|
- An octahedral complex is prepared by mixing CoCl3 and NH3 in the molar...
Text Solution
|
- Identify the incorrect characteristic of carbenes,: CR2
Text Solution
|
- Which is the correct stability order for the given carbonium ions? A.M...
Text Solution
|
- Among the following species, how many are aromatic in nature?
Text Solution
|
- Peroxide plays a vital role in producing
Text Solution
|
- Products formed in the above reaction are result of
Text Solution
|
- An aldehyde reacts with KCN to form cyanohydrin. In this reaction
Text Solution
|
- Why do alkenes prefer to undergo electrophilec addition reaction while...
Text Solution
|
- What are homogeneous catalysts? state one example
Text Solution
|
- Why are alkyl halides insoluble in water?
Text Solution
|
- The following reaction falls under the category of
Text Solution
|
- The intermediate formed in the electrophilic addition of HBr to propen...
Text Solution
|
- How many elimination products are formed when the given dibrom...
Text Solution
|