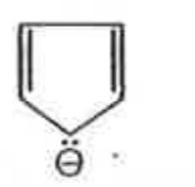
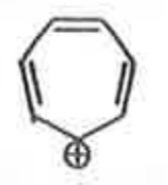
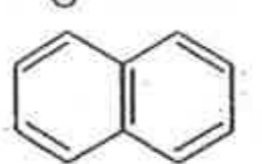
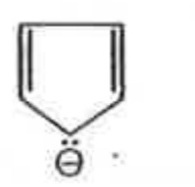
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-MOCK TEST 33-Example
- choose the incorrect statement from the following
Text Solution
|
- which among the following statement is incorrect regarding the product...
Text Solution
|
- An example of a antiaromatic species is
Text Solution
|
- Consider the following reaction , the compounds P and Q are respective...
Text Solution
|
- Number of sp hybridised carbon atoms in But-2-yne is
Text Solution
|
- Give reasons : C–X bond length in halobenzene is smaller than C–X bond...
Text Solution
|
- Total number of pi-electrons in benzene is
Text Solution
|
- Total number of hydrogen molecules required to form ethane from ethyne...
Text Solution
|
- In anthrcene ,number of pi electrons is equal to x. the value of x is
Text Solution
|
- The colour chage observed when excess ethyne is passed through the sol...
Text Solution
|
- Major product is
Text Solution
|
- consider the given box the total number of gases which are responsible...
Text Solution
|
- For clear water ,its BOD should be less than
Text Solution
|
- Consider the following reaction X and Y respectively are
Text Solution
|
- All the following are the effects of depletion of ozone layer, except
Text Solution
|
- IUPAC name of K3[Fe(C2O4)3] is?
Text Solution
|
- Which of the following is not an example of organochlorine which shows...
Text Solution
|
- Product P is
Text Solution
|
- Which of the following gases combines with haemoglobin to form a very ...
Text Solution
|
- In an electrophilic aromatic substitution reaction, the nitro group is...
Text Solution
|