A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-MOCK_TEST_17-Example
- Two substance of same size are made of same material but one is hollow...
Text Solution
|
- A body cools in 10 minutes from 60^@C to 40^@C. What will be its tempe...
Text Solution
|
- If the temperature of the body is increases from 27°C to 327°C then wa...
Text Solution
|
- Which of the following law states that "good absorbes of heat are good...
Text Solution
|
- The ratio of time taken by ice on the surface of ponds or lakes to bec...
Text Solution
|
- For perfectly black body emissivity (e) is
Text Solution
|
- A body cools in 3 minute from 90°C to 80°C. The temperature reduce to ...
Text Solution
|
- If there is no gravity i.e. acceleration due to gravity is zero then w...
Text Solution
|
- Which of the following statement is correct
Text Solution
|
- A black body, at temperature T K emits radiation at the rate of 81 W/m...
Text Solution
|
- If 2 is a zero of the polynomial ax^2-2x then the value of 'a' is ......
Text Solution
|
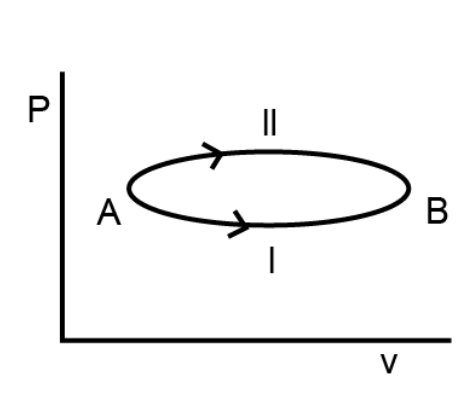
- Two path 1 and 2 are shown in figure. If the change in internal energi...
Text Solution
|
- The work done by the gas in the process shown in given P-V diagram is
Text Solution
|
- For a gaseous state if heat supplied to the system is 100 J and work d...
Text Solution
|
- Internal energy of an ideal gas changes with change in its
Text Solution
|