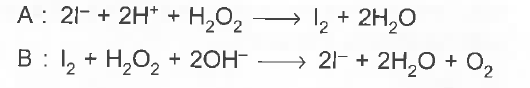A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-TEST 5-EXAMPLE
- Choose the incorrect statement.
Text Solution
|
- Elements of which of the following group(s) of periodic table do ...
Text Solution
|
- Consider the reaction H2O2 is
Text Solution
|
- Which of the following is electron deficient-hydride?
Text Solution
|
- Match the Column-I to Column-II
Text Solution
|
- Choose the incorrect statement.
Text Solution
|
- Equivalent mass of KMnO4 in acidic and basic medium respectively [M = ...
Text Solution
|
- Identify the compound in which 'S' has highest oxidation state.
Text Solution
|
- Given : Identify the cell which will give maximum EMF^o
Text Solution
|
- What is the average oxidation state of oxygen In S2O8^(2-)
Text Solution
|
- MnO(4)^(2-) undergoes disproportionation reaction in acidic medium but...
Text Solution
|
- Identity the disproportionation reaction.
Text Solution
|
- Which of the following arrangements represent increasing oxidation num...
Text Solution
|
- In which of the following compounds, an element exhibits two different...
Text Solution
|
- Consider the following reactions. I. 2PbO + 4HCl to 2PbCl2 + 2H2O ll....
Text Solution
|
- Choose the non-redox reaction.
Text Solution
|
- The most basic oxide among the following is
Text Solution
|
- Among the following the least soluble sulphate is
Text Solution
|
- Thermal stability of hydrides of alkali metals
Text Solution
|
- In the manufacture of sodium hydroxide, by product obtained is
Text Solution
|