A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
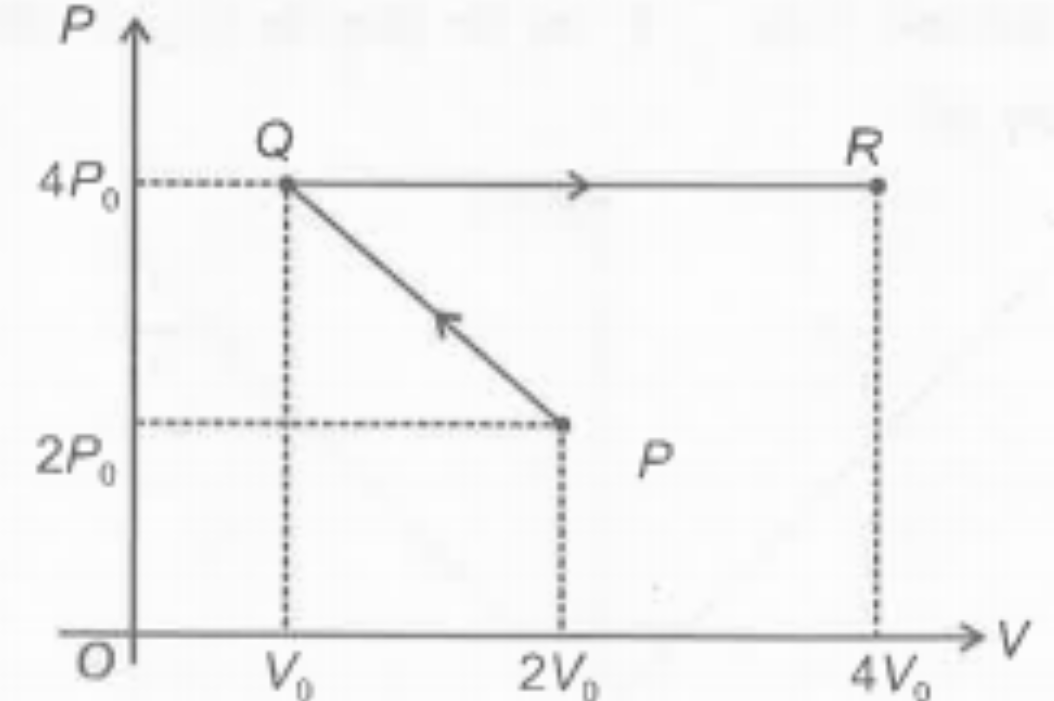
- An ideal gas undergoes a process P→Q→R as shown in pressure (p) - volu...
Text Solution
|
- An ideal gas has initial volume V and pressure p. In doubling its volu...
Text Solution
|
- One mole of an ideal gas has an interal energy given by U=U(0)+2PV , w...
Text Solution
|
- P - V diagram of an ideal gas is as shown in figure. Work done by the ...
Text Solution
|
- An ideal gas underoges cyclic process of ABCDA as shown in Given P-V d...
Text Solution
|
- An ideal gas undergoes cyclic process ABCDA as shown in givend p-V dia...
Text Solution
|
- P – V graph of an ideal gas is as shown in the diagram . Work done by ...
Text Solution
|
- An ideal gas undergoes cyclic process ABCDA as shown in given P - V di...
Text Solution
|
- P-V diagram of an ideal gas is as shown in figure. Work done by the ga...
Text Solution
|