Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Important Questions|28 VideosATOMIC STRUCTURE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS |39 VideosCHEMICAL EQUILIBRIUM AND ACIDS BASES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |31 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ATOMIC STRUCTURE-LONG ANSWER QUESTIONS
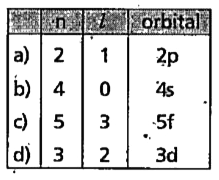
- Using s, p, d , f notations , describe the orbital with the following ...
Text Solution
|
- Explain Rutherford 's nuclear of an atom . What are its drawbacks ?
Text Solution
|
- Write Planck's equation .
Text Solution
|
- What are the postulates of Bohr's model of hydrogen atom ? Discuss the...
Text Solution
|
- Explain the success of Bohr's theory for hydrogen atom.
Text Solution
|
- What are the consequences that lead to the development of quantum mech...
Text Solution
|
- What are the main features of quantum mechanical model of an atom?
Text Solution
|
- What are the limitation of Bohr's model of an atom?
Text Solution
|
- What are the evidence in favour of dual behaviour of electron?
Text Solution
|
- How are the quantum numbers n , 1 m(1) for hydrogen atom are obtained...
Text Solution
|
- Explain the dual behaviour of matter. Discuss its significance to micr...
Text Solution
|
- What are characteristics of electromagnetic waves ?
Text Solution
|
- Define atomic orbital . Explain the shapes of s, p and d orbitals with...
Text Solution
|
- Explain diagrammatically the boundary surfaces for three 2p orbitals a...
Text Solution
|
- IIIustrate the reaasons for the stability of completey filled and half...
Text Solution
|
- Explain emission and absorption spectra. Discuss the general descripti...
Text Solution
|