Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS |21 VideosATOMIC STRUCTURE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |15 VideosATOMIC STRUCTURE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Important Questions|28 VideosCHEMICAL EQUILIBRIUM AND ACIDS BASES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |31 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ATOMIC STRUCTURE-VERY SHORT ANSWER QUESTIONS
- What is the complete symbol for the atom with the given atomic number ...
Text Solution
|
- Draw the shape of d(z^(2)) orbital .
Text Solution
|
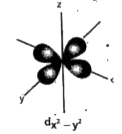
- Draw the shape of d(x^(2)-y^(2)) orbital .
Text Solution
|
- What is the frequency of radiation of wavelenth 600 nm ?
Text Solution
|
- What is Zeeman effect ?
Text Solution
|
- What is Stark effect ?
Text Solution
|
- To which element does the following electronic configuration correspon...
Text Solution
|
- To which element does the following electronic configuration correspon...
Text Solution
|
- To which element does the following electronic configuration correspon...
Text Solution
|
- To which element does the following electronic configuration correspon...
Text Solution
|
- Electrons are emitted with zero velocity from a metal surface when it ...
Text Solution
|
- State and explain Pauli's exclusion principle.
Text Solution
|
- What is Aufbau principle ?
Text Solution
|
- What is Hund's rule ?
Text Solution
|
- Explain Heisenberg's uncertainty principle .
Text Solution
|
- What is the wavelength of an electron moving with a velocity of 2.0xx1...
Text Solution
|
- An atomic orbital has n = 2 , what are the possible valuse of l and m...
Text Solution
|
- Which of the following orbitals are possible ? (2s,1p,3f,2p)
Text Solution
|
- The static electric charge on the oil drop is -3.2044xx10^(-19)C . Ho...
Text Solution
|
- Arrange the following type of radiation in increasing order of frequen...
Text Solution
|