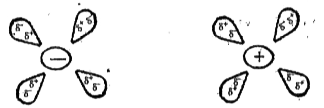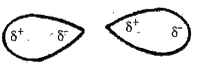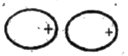Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER GASES AND LIQUIDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS|26 VideosORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise IMPORTANT QUESTIONS|42 VideosSTOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Important Question |39 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-STATES OF MATTER GASES AND LIQUIDS -LONG ANSWER QUESTIONS
- Write notes on Intermolecular forces
Text Solution
|
- State Boyle's law, Charles' law and Avogadro's law and derive ideal ga...
Text Solution
|
- Write notes on diffusion of Gases.
Text Solution
|
- State and explain Dalton's law of partial pressures.
Text Solution
|
- Write the postulates of kinetic Molecular Theory of Gases.
Text Solution
|
- Derive the gas laws from the kinetic gas equation.
Text Solution
|
- Explain Maxwell-Boltzmann distribution curves of molecular speeds and ...
Text Solution
|
- What are the reasons for deviations from ideal gas behaviour ?
Text Solution
|
- Derive and expalin van der Waals equation of state
Text Solution
|
- Explain the liquefication of gases.
Text Solution
|
- Write notes on the following properties of liquids a) Vapour pressur...
Text Solution
|
- Write notes on Intermolecular forces
Text Solution
|
- State Boyle's law, Charles' law and Avogadro's law and derive ideal ga...
Text Solution
|
- Write notes on diffusion of Gases.
Text Solution
|
- State and explain Dalton's law of partial pressures.
Text Solution
|
- Write the postulates of kinetic Molecular Theory of Gases.
Text Solution
|
- Derive the gas laws from the kinetic gas equation.
Text Solution
|
- Explain Maxwell-Boltzmann distribution curves of molecular speeds and ...
Text Solution
|
- A real gas deviates most from ideal behaviour at
Text Solution
|
- Derive the van der Waals equation of state. Explain the importance of ...
Text Solution
|