Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM AND ACIDS BASES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |31 VideosCHEMICAL EQUILIBRIUM AND ACIDS BASES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS |40 VideosATOMIC STRUCTURE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |15 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Long Answer Questions|24 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-CHEMICAL EQUILIBRIUM AND ACIDS BASES - SHORT ANSWER QUESTIONS
- Derive the relation between K(p) and K(c) for the equilibrium reaction...
Text Solution
|
- Define equilibrium constant. Write the equilibrium constant expression...
Text Solution
|
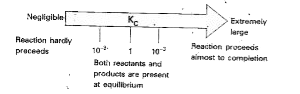
- How does the values of equilibrium constant predict the extent of reac...
Text Solution
|
- State law of chemical equilibrium? What is K(c) for the following equi...
Text Solution
|
- Why sealed soda water bottle on opening shows the evolution of gas wit...
Text Solution
|
- Explain the significance of : a very large value of K
Text Solution
|
- Explain the significance of : a very small value of K
Text Solution
|
- Explain the significance of : a value of K of about 1.0
Text Solution
|
- Why is it useful to compare Q with K? What is the situation when ...
Text Solution
|
- Why is it useful to compare Q with K? What is the situation when ...
Text Solution
|
- Why is it useful to compare Q with K? What is the situation when ...
Text Solution
|
- For the reaction Cl(2)(g)+F(2)(g)hArrClF(g),K(c)=19.9 What will happ...
Text Solution
|
- Predict which of the following reactionn will have appreciable concent...
Text Solution
|
- Predict which of the following reactionn will have appreciable concent...
Text Solution
|
- Predict which of the following reactionn will have appreciable concent...
Text Solution
|
- How to recognise the conditions under which changes in pressure would ...
Text Solution
|
- What property of a reaction can be used to predict the effect of a cha...
Text Solution
|
- Does the number of moles of reaction products increase, decrease, or r...
Text Solution
|
- Does the number of moles of reaction products increase, decrease, or r...
Text Solution
|
- Which of the following reactions will get affected by increasing the p...
Text Solution
|