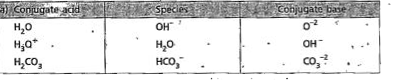
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM AND ACIDS BASES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |31 VideosCHEMICAL EQUILIBRIUM AND ACIDS BASES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS |40 VideosATOMIC STRUCTURE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |15 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Long Answer Questions|24 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-CHEMICAL EQUILIBRIUM AND ACIDS BASES - SHORT ANSWER QUESTIONS
- The species H(2)O,HCO(3)^(-),HSO(4)^(-) and NH(3) can act both as Bron...
Text Solution
|
- Write equation that showss H(2)PO(4)^(-) acting both as an acid and as...
Text Solution
|
- Write the conjugate acid and conjugate base of each of the following: ...
Text Solution
|
- Identity and label the Bronsted acid and its conjugate base,te Bronste...
Text Solution
|
- Classify the species AlCl(3), NY(3), Mg^(+2) and H(2)O into Lewis acid...
Text Solution
|
- What are the strengths of conjuate bases of a strong acid and a weak a...
Text Solution
|
- What are the strengths of conjuate acids of a strong base and weak bas...
Text Solution
|
- Define ionic product of water. What is the value at room temperature?
Text Solution
|
- Define pH. pH cannot be calculated directly from the molar concentrati...
Text Solution
|
- Write equations to show the step wise ionization of the polyprotic aci...
Text Solution
|
- Explain how acid strength changes among i. the hydrides of the group...
Text Solution
|
- Explain how acid strength changes among i. the hydrides of the group...
Text Solution
|
- Justifyi the statement that water behaves like an acid an also like ba...
Text Solution
|
- What is common ion effect? Illustrate.
Text Solution
|
- Define solubility product. Write solubility product expressiions for t...
Text Solution
|
- Define solubility product. Write solubility product expressions for th...
Text Solution
|
- Give the classification of salts. What types of salts undergo hydrolys...
Text Solution
|
- What must be true of value of DeltaG^(@) for a reaction if Kgt1
Text Solution
|
- What must be true of value of DeltaG^(@) for a reaction if K=1
Text Solution
|
- What must be true of value of DeltaG^(@) for a reaction if K=1
Text Solution
|