Similar Questions
Explore conceptually related problems
Recommended Questions
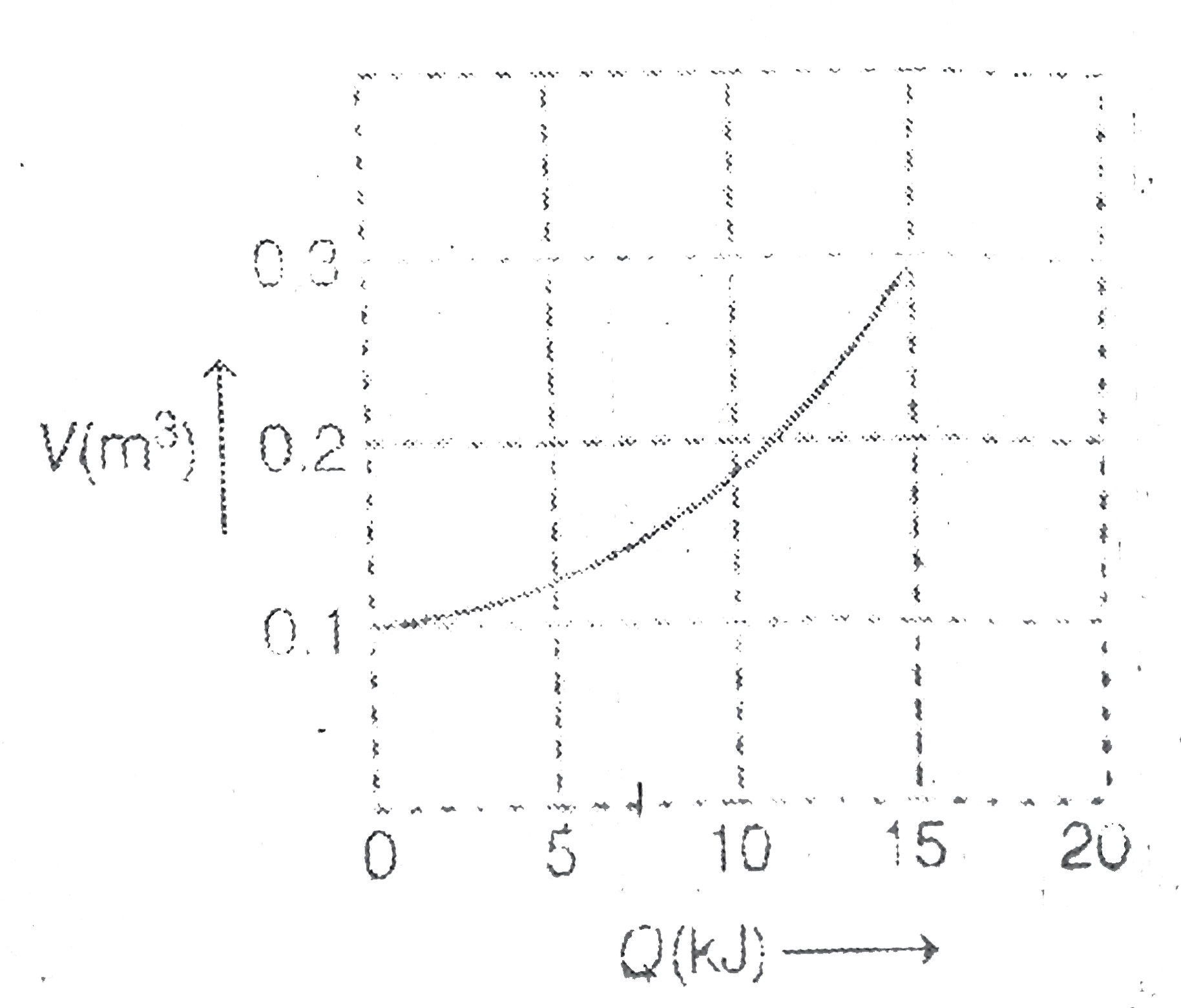
- Consider isothermal expansion of 0.5 mole of an ideal gas when Q amoun...
Text Solution
|
- Calcualte q, w, DeltaU, and DeltaH for the reversible isothermal expan...
Text Solution
|
- Suppose 0.5 moles of an ideal gas undergoes an isothermal expansion as...
Text Solution
|
- A cyclinder contains 0.5 mole of an ideal gas at 310K . As the gas exp...
Text Solution
|
- The entropy of v = 4.0 moles of an ideal gas increases by Delta S = 23...
Text Solution
|
- Consider isothermal expansion of 0.5 mole of an ideal gas when Q amoun...
Text Solution
|
- Calculate q,w, DeltaU and DeltaH for the reversible isothermal expansi...
Text Solution
|
- For the reversible isothermal expansion of one mole of an ideal gas at...
Text Solution
|
- One mole of an ideal gas requires 207 J of heat to raise the temperatu...
Text Solution
|