Similar Questions
Explore conceptually related problems
Recommended Questions
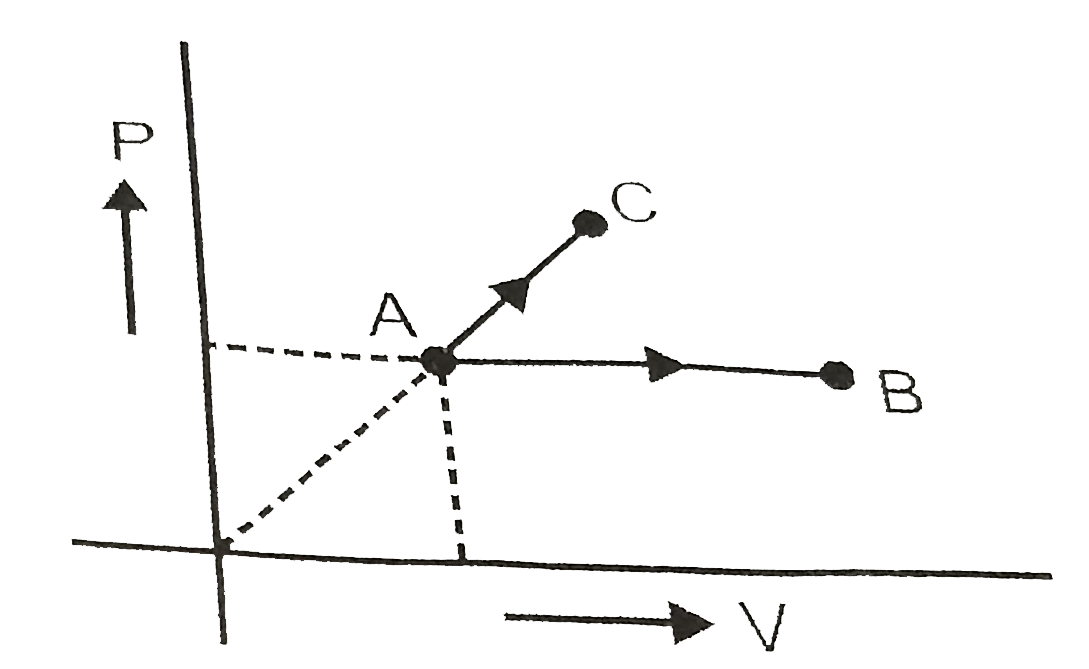
- One mole ideal monoatomic gas is heated according to path AB and AC. ...
Text Solution
|
- Figure. Shows an ideal gas changing its state A to state C by two diff...
Text Solution
|
- An ideal gas of mass m in a state A goes to another state B via three ...
Text Solution
|
- The given figure shown a change of state A to state C by two paths ABC...
Text Solution
|
- In the figure an ideal gas changes is state from state A to state C by...
Text Solution
|
- One mole of an ideal monoatomic gas is heated according to path AB and...
Text Solution
|
- सारणिक |(b^(2),ab,b,c,bc,ac),(ab,a^(2),a,b,b^(2),ab),(bc,ac,c,a,ab,a^(...
Text Solution
|
- One mole of ideal monoatomic gas is taken round the cyclic process as ...
Text Solution
|
- One mole ideal monoatomic gas is heated according to path AB and AC. I...
Text Solution
|