Similar Questions
Explore conceptually related problems
Recommended Questions
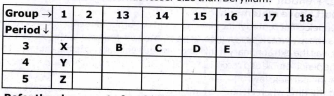
- Write the electronic configuration of the elements B and E.
Text Solution
|
- लेन्थेनाइड तत्त्वों के इलेक्ट्रॉनिक विन्यास लिखिए।
Text Solution
|
- सल्फर तत्वों के इलेक्ट्रॉनिक विन्यास लिखिए-
Text Solution
|
- आर्गन तत्वों के इलेक्ट्रॉनिक विन्यास लिखिए-
Text Solution
|
- क्रोमियम तत्वों के इलेक्ट्रॉनिक विन्यास लिखिए-
Text Solution
|
- क्रिप्टॉन तत्वों के इलेक्ट्रॉनिक विन्यास लिखिए-
Text Solution
|
- Write electronic configuration of some elements.
Text Solution
|
- Write the electronic configuration of following elements: (a) Copper ...
Text Solution
|
- Write the anomalous electronic configuration of elements.
Text Solution
|