Topper's Solved these Questions
THE AGE OF ADOLESCENCE
NCERT TELUGU|Exercise Think and Discuss|5 VideosTHE AGE OF ADOLESCENCE
NCERT TELUGU|Exercise Improve your learning |16 VideosTHE AGE OF ADOLESCENCE
NCERT TELUGU|Exercise Improve your learning |16 VideosSTORY OF MICROORGANISMS
NCERT TELUGU|Exercise MEDICINE ORIENTED MATERIAL|10 VideosTHE WORLD OF MICRO ORGANISMS
NCERT TELUGU|Exercise EXERCISE|30 Videos
Similar Questions
Explore conceptually related problems
NCERT TELUGU-THE AGE OF ADOLESCENCE-EXERCISE
- What can you suggest to your classmates to keep himself/herself clean ...
Text Solution
|
- If you have chance to talk with a doctor, what questions you would ask...
Text Solution
|
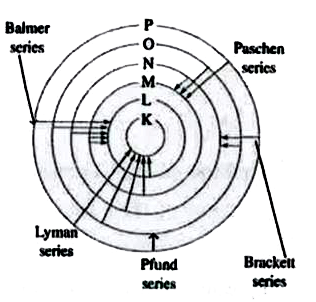
- The only electron in the hydrogen atom resides under ordinary conditio...
Text Solution
|
- Write five suggestions to improve the performance of Red Ribbon club o...
Text Solution
|
- Prepare a five minutes speech or a presestation about the information ...
Text Solution
|
- Nature prepares human body to reproduce her generations. What do you t...
Text Solution
|
- You know that early marriage is a social taboo. Prepare some slogans t...
Text Solution
|
- 13 years old Swaroop always think of his height. Can he improve his he...
Text Solution
|
- Observe some of your friends and note their characters in the follow...
Text Solution
|
- What are your expectations about your parents and teachers?
Text Solution
|
- Often you may have experienced that if you have tension for some reaso...
Text Solution
|
- How you noticed that you are growing?
Text Solution
|
- Have you reached the age of "Adolescence"? Is mustache growing on yo...
Text Solution
|
- Have you reached the age of "Adolescence"? Did your voice change?
Text Solution
|
- Have you reached the age of "Adolescence"? Are hairs growing under a...
Text Solution
|
- How many lattice points are there in one unit cell of face centered cu...
Text Solution
|
- Have you reached the age of "Adolescence"? Are you taking care of yo...
Text Solution
|
- Have you reached the age of "Adolescence"? Are you feeling shy when...
Text Solution
|
- Are you interested to play with opposite sex, which have you done earl...
Text Solution
|
- Which methods do you suggest to conserve soil ?
Text Solution
|