Similar Questions
Explore conceptually related problems
Recommended Questions
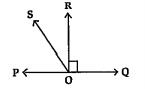
- In the given figure oversetharr( PQ) is a line. Ray oversetharr(OR) is...
Text Solution
|
- If A, B, C are the points (0, 4, 1), (2, 3, -1), (4, 5, 0) respectivel...
Text Solution
|
- IF the d.r.'s of oversetharr(OA),oversetharr(OB) are (1, -2, -1), (3, ...
Text Solution
|
- Consider the line oversetharr(PQ) given below and find whether the gi...
Text Solution
|
- Consider the line oversetharr(PQ) given below and find whether the gi...
Text Solution
|
- Consider the line oversetharr(PQ) given below and find whether the gi...
Text Solution
|
- Consider the line oversetharr(PQ) given below and find whether the gi...
Text Solution
|
- Write 'T' for true and 'F' for false in case of each of the following ...
Text Solution
|
- In the given figure bar(PQ) is a line. Ray bar(OR) is perpendicular t...
Text Solution
|