Similar Questions
Explore conceptually related problems
Recommended Questions
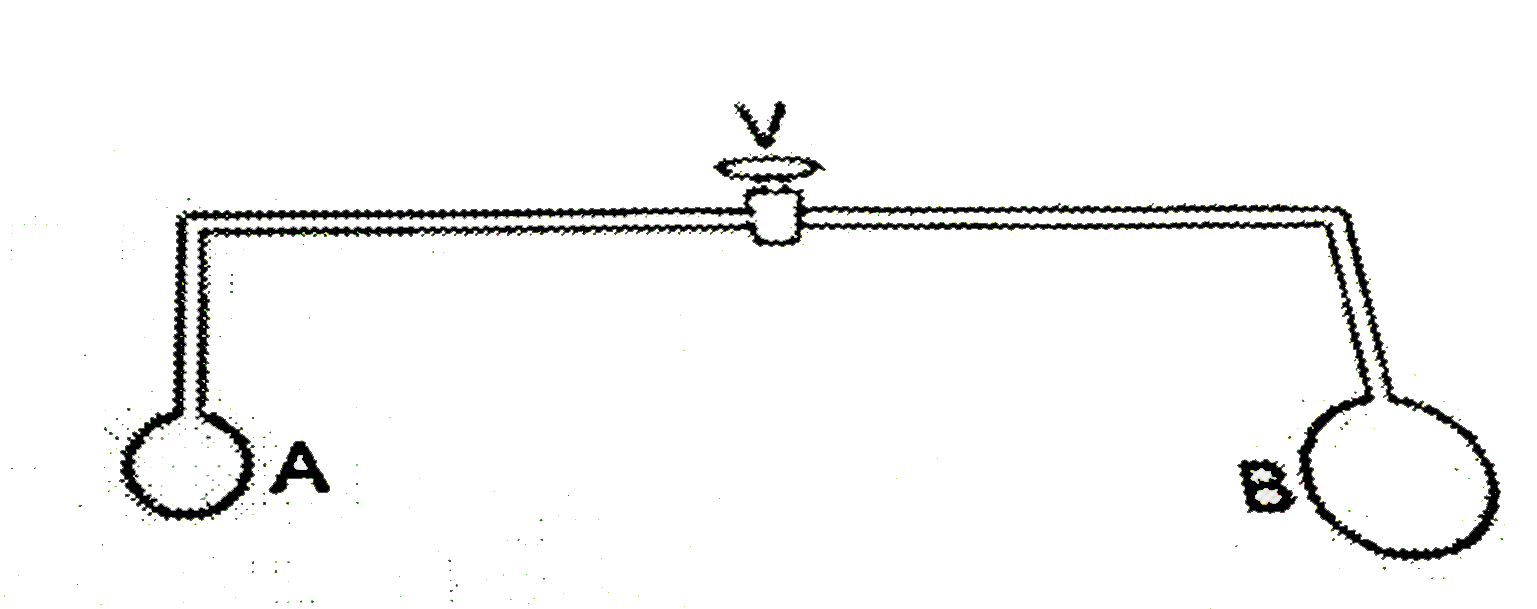
- The value V in the bent tube is initially kept closed. Two soap bubble...
Text Solution
|
- Two unequal soap bubbles are formed one on each side of a tube closed ...
Text Solution
|
- Two soap bubbles A and B of different diameters are blown at the two e...
Text Solution
|
- Tow soap bubbles A and B are formed at the two open ends of a tube. Th...
Text Solution
|
- Two soap bubbles are formed on the end of the ends of the tubes as sho...
Text Solution
|
- The value V in the bent tube is initially kept closed. Two soap bubble...
Text Solution
|
- A and B are two soap bubbles. Bubble A is larger than B. If these are ...
Text Solution
|
- If the volume of two soap bubbles are V and 8V bubbles is
Text Solution
|
- Two unequal soap bubbles are formed one on each side of a tube closed ...
Text Solution
|