Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
V PUBLICATION-Structure of Atom-QUESTION BANK
- Find the atomic number of the atom which is given below. (16) overset(...
Text Solution
|
- The representation of the Bohr atom model of an element is given below...
Text Solution
|
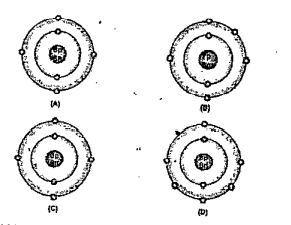
- Bohr models of atoms A, B,C,D are given (Symbols are not real). a) W...
Text Solution
|
- a) What is the difference between atomic number and mass number. b) If...
Text Solution
|
- Notice the three atomș given below: underset(17) overset(34) \Q , und...
Text Solution
|
- A,B, C, D and E five elements (These symbols are not real? underset (...
Text Solution
|
- Given below are the three isotopes of Hydrogen. underset(1) overset(1...
Text Solution
|
- Complete the table.
Text Solution
|
- Draw the Bohr model of argon atom. (Atomic numberAr=18 )
Text Solution
|
- Give reason for the following. a) atoms are electrically, neutral. b)...
Text Solution
|
- In the atom of an element 7 electrons are present in the M shell. : Th...
Text Solution
|
- An atom of aluminium (Al) has 13 electrons and 14 neutrons in it. a) ...
Text Solution
|
- Some details of the atoms X and Y are given below. a) Find the mass nu...
Text Solution
|
- Atomic number of an atom Z=17, A=35. a) Find the number of protons, ...
Text Solution
|
- Symbols (not real symbols) of some.atoms are given. underset(8) overs...
Text Solution
|
- True or false? . a) All particles in the atom have charge. b) Elect...
Text Solution
|
- Look at their properties in the table. Try to fill in the blanks.
Text Solution
|
- Some questions related to atoms are given below. Choose correct word f...
Text Solution
|
- An element is represented as follows (Symbol is not real) underset(13...
Text Solution
|
- The mass number of an element is 23 and its atomic number is 11 a) Fi...
Text Solution
|