Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
V PUBLICATION-CHEMICAL BONDING-QUESTION BANK
- a) Write the chemical formula of barium chloride b) Write the chemic...
Text Solution
|
- Draw the electron dot diagram of chemical bonds in ethane (C2H6).
Text Solution
|
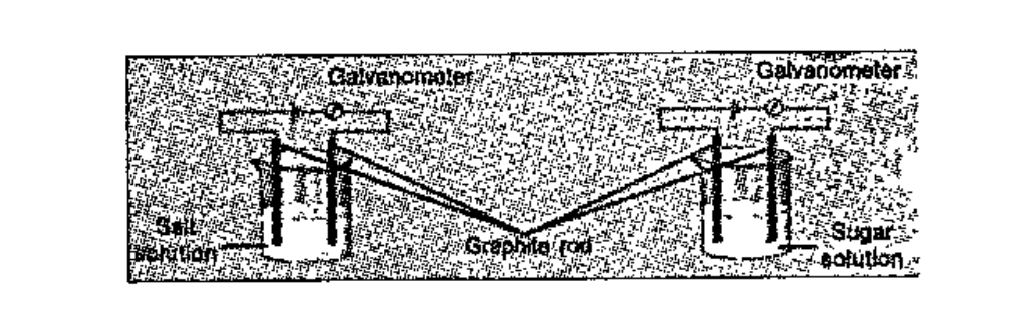
- Perform the experiment arranging the apparatus as shown in figure. R...
Text Solution
|
- P,Q,R,S are four elements. THeir atomic numbers are 8, 17, 12 and 16 r...
Text Solution
|
- The capacity of an atom to take part in chemical reaction is called.
Text Solution
|
- Why do atoms take part in CHEMICAL BONDING?
Text Solution
|
- Atoms donate electron and changed to ......ions.
Text Solution
|
- Which of the follwing is a monoatomic molecule? (Oxygen, Argon, Chlori...
Text Solution
|
- The compound formed when magnesium burns in oxygen is
Text Solution
|
- FInd the relation and fill up suitably The CHEMICAL BONDING formed b...
Text Solution
|
- The element which does not take part in CHEMICAL BONDING are ........
Text Solution
|
- Helium is an example for .........molecule
Text Solution
|
- In which state are most ionic compounds found?
Text Solution
|
- Write an example for covalent compound in liquid state.
Text Solution
|
- The bonding in calcium chloride is .............
Text Solution
|
- If the difference in electronegativity between two constituent element...
Text Solution
|
- The molecular formula of aluminium chloride is AlCl3. Find the valenci...
Text Solution
|
- THe atomic number of fluorine is 9. Draw the electron dot diagram of t...
Text Solution
|
- Find the relation and fill up suitably: Hydrogen: Single Bond Nitr...
Text Solution
|
- Identify the wrong and the correct statemens a) Covalent compounds a...
Text Solution
|
 .
.