Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
V PUBLICATION-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-QUESTION BANK
- What do you understand by isoelectronic species?Name a species that wi...
Text Solution
|
- N^(3-),O^(2-),F^-,Na^+, Mg^(2+),Al^(3+) what is comman in them ?
Text Solution
|
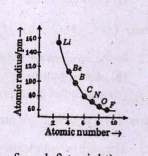
- A graph of aromic radius versus atomic number is given blow: Account f...
Text Solution
|
- What is the significance of the terms - isolated gaseous atom and grou...
Text Solution
|
- Energy of an electron in the ground state of hydrogen atom is -2.18 xx...
Text Solution
|
- Among the second period elements the actual ionisation enthaply are in...
Text Solution
|
- How would you explain that the first ionisation energy of Na is lawer ...
Text Solution
|
- What are the main factors due to which the ionisation enthalpy of the ...
Text Solution
|
- The first IE (Kj mol^(-1))of group 13 elements are B/801 Al/577 Ga...
Text Solution
|
- Which of the following pairs would have more negative electron gain en...
Text Solution
|
- Would you expect the second electron gain enthalpy O as positive, more...
Text Solution
|
- What is the basic difference between the terms electron gain enthalpy ...
Text Solution
|
- How would you react to the statement that the electron(eg)ativity of '...
Text Solution
|
- Discribe the theory associated with the radius of an atom as it gains ...
Text Solution
|
- Would you expect the first IE of two isotopes of the same elements to ...
Text Solution
|
- What are the mojor differece between metals and non metals ?
Text Solution
|
- Use the periodic table to answer the.following questions? a. Identify...
Text Solution
|
- The inceasing order of reactivity among group 1 elements is Li lt Na l...
Text Solution
|
- Write the general outer electronic configuration of 's', 'p, d' and 'f...
Text Solution
|
- Assign the position of the element having outẹr electronic configurati...
Text Solution
|