Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
V PUBLICATION-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-QUESTION BANK
- Write the general outer electronic configuration of 's', 'p, d' and 'f...
Text Solution
|
- Assign the position of the element having outẹr electronic configurati...
Text Solution
|
- The first IE and second IE (Kj mol^(-1)) and daltaegH(kJ mol^(-1)) of...
Text Solution
|
- Predict the formulae of stable binary compounds that would be the form...
Text Solution
|
- In the modern periodic table, the period indicates the value of
Text Solution
|
- Which of the following statements related to the modern periodic table...
Text Solution
|
- Anything that influences the valence electrons will affect the chemist...
Text Solution
|
- The size of iso electronic species F-Ne and Na^+ is affected by
Text Solution
|
- Which one of the following statements is incorrect in relation to ioni...
Text Solution
|
- Considering the elements B,Al,Mg and K, the correct order of their me...
Text Solution
|
- Considering the elements, 'B, C, N, F', and 'Si', the correct order of...
Text Solution
|
- considering the elements F,Cl, O and N, the correct order their chemic...
Text Solution
|
- Atomic numbers of two elements 'A' and 'B' are 31 and 41 respectively....
Text Solution
|
- A group of ions are given below: Na^+, Al^3+, O^2-, Ca^2+, Mg^2+, ...
Text Solution
|
- Which of the following will have the most negative electron gain enth...
Text Solution
|
- Predict the formulae of compounds which might be formed by the followi...
Text Solution
|
- The elements Z=107 and Z=109 have been made recently, element Z=108 ha...
Text Solution
|
- Two elements Cand D have atomic numbers 36 and 58 respectively. On the...
Text Solution
|
- Which of the following species will have the largest and the smallest ...
Text Solution
|
- The first ionization enthalpy (IE) of the third period elements 'Na, M...
Text Solution
|
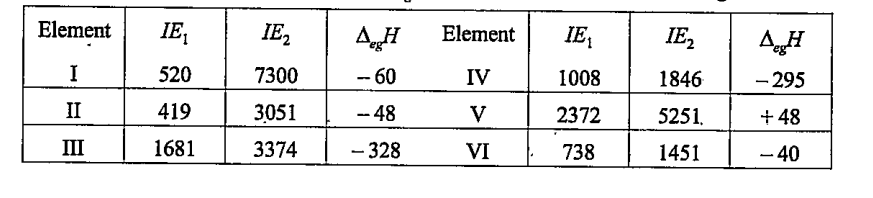 Which of the above is likely to be the least reactive element
Which of the above is likely to be the least reactive element