Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-REVISION DPP-All Questions
- The degree of freedom per molecule for a gas is 6. At constant pressur...
Text Solution
|
- 5 moles of nitrogen gas are enclosed in an adiabatic cylindrical vesse...
Text Solution
|
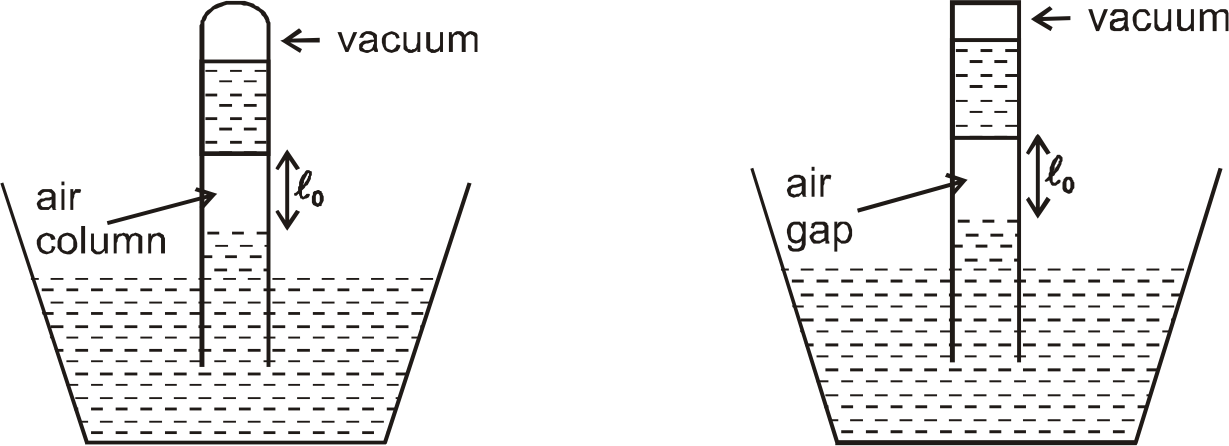
- At the middle of the mercury barometer tube there is a little column o...
Text Solution
|
- One mole of an ideal gas (y=1.4) at 500K, is filled in an adiabatic cy...
Text Solution
|
- The current density bar(J) inside a long, solid cylindrical wire of ra...
Text Solution
|
- The basic idea of Quantum Mechanics is that motion in any system is qu...
Text Solution
|
- The basic idea of Quantum Mechanics is that motion in any system is qu...
Text Solution
|
- The basic idea of Quantum Mechanics is that motion in any system is qu...
Text Solution
|
- The fixed non-conducting cylinder shown in figure has a noconducting h...
Text Solution
|
- The fixed non-conducting cylinder shown in figure has a noconducting h...
Text Solution
|
- The fixed non-conducting cylinder shown in figure has a noconducting h...
Text Solution
|
- Sand is droped vetically downward at the constant rate mu kg//s on a c...
Text Solution
|
- Sand is droped vetically downward at the constant rate mu kg//s on a c...
Text Solution
|
- Sand is droped vetically downward at the constant rate mu kg//s on a c...
Text Solution
|
- One mole of an ideal gas is contained in a perfectly insulating cylind...
Text Solution
|
- One mole of an ideal gas is contained in a perfectly insulating cylind...
Text Solution
|
- One mole of a diatomic gas is heated under a "kibolinsky process" in w...
Text Solution
|
- One mole of a diatomic gas is heated under a "kibolinsky process" in w...
Text Solution
|
- One mole of a diatomic gas is heated under a "kibolinsky process" in w...
Text Solution
|
- In each situation of column-I,a process ArarrBrarrC is given for an id...
Text Solution
|