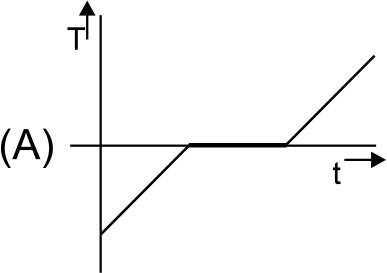
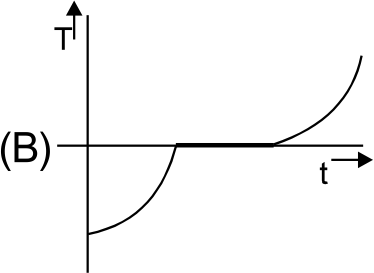
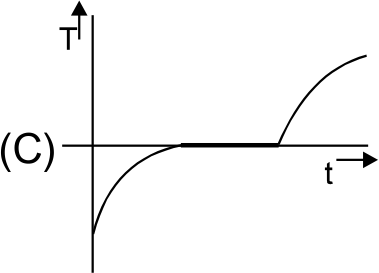
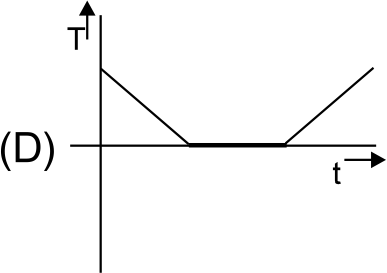
A
B
C
D
Text Solution
AI Generated Solution
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-REVISION DPP-All Questions
- An ideal gas consists of a large number of identical molecules. Absolu...
Text Solution
|
- Let the wavelength at which the the spectral emissive power of a black...
Text Solution
|
- If specific ehat capacity of a substance in solid and liquid state is ...
Text Solution
|
- A resistor has initial resistance R(0) at 0^(@)C now it is connected t...
Text Solution
|
- A sphere and a cube of same material and same total surface area are p...
Text Solution
|
- Water of mass m(2) = 1 kg is contained in a copper calorimeter of mass...
Text Solution
|
- An insulated chamber at a height h above the earth's surface and maint...
Text Solution
|
- A force of constant magnitude F acts on a particle moving in a plane s...
Text Solution
|
- All the rods have same conductance 'K' and same area of cross section ...
Text Solution
|
- A wire is bent in a parabolic shape followed by equation x=4y^(2) cons...
Text Solution
|
- Equal volumes of water and alcohol when put in similar calorimeters ta...
Text Solution
|
- An isosceles triangles is formed with a thin rod of length l(1) and co...
Text Solution
|
- A steel wire is rigidly fixed along diameter of aluminium ring of radi...
Text Solution
|
- The temperature of isolated black body falls from T(1) "to "T(2) in ti...
Text Solution
|
- Two containers having boiling water and ice are connected through a co...
Text Solution
|
- A rod of uniform cross-section but non-uniform thermal conductivity wh...
Text Solution
|
- A vessel is partly filled with a liquid. Coefficients of cubical expan...
Text Solution
|
- The ends of a rod of uniform thermal conductivity are maintained at di...
Text Solution
|
- A straight nicrome wire is initially at room temperature 20^(@)C it is...
Text Solution
|
- 2 kg of ice at -20^(@)C is mixed with 5kg of water at 20^(@)C. The wat...
Text Solution
|