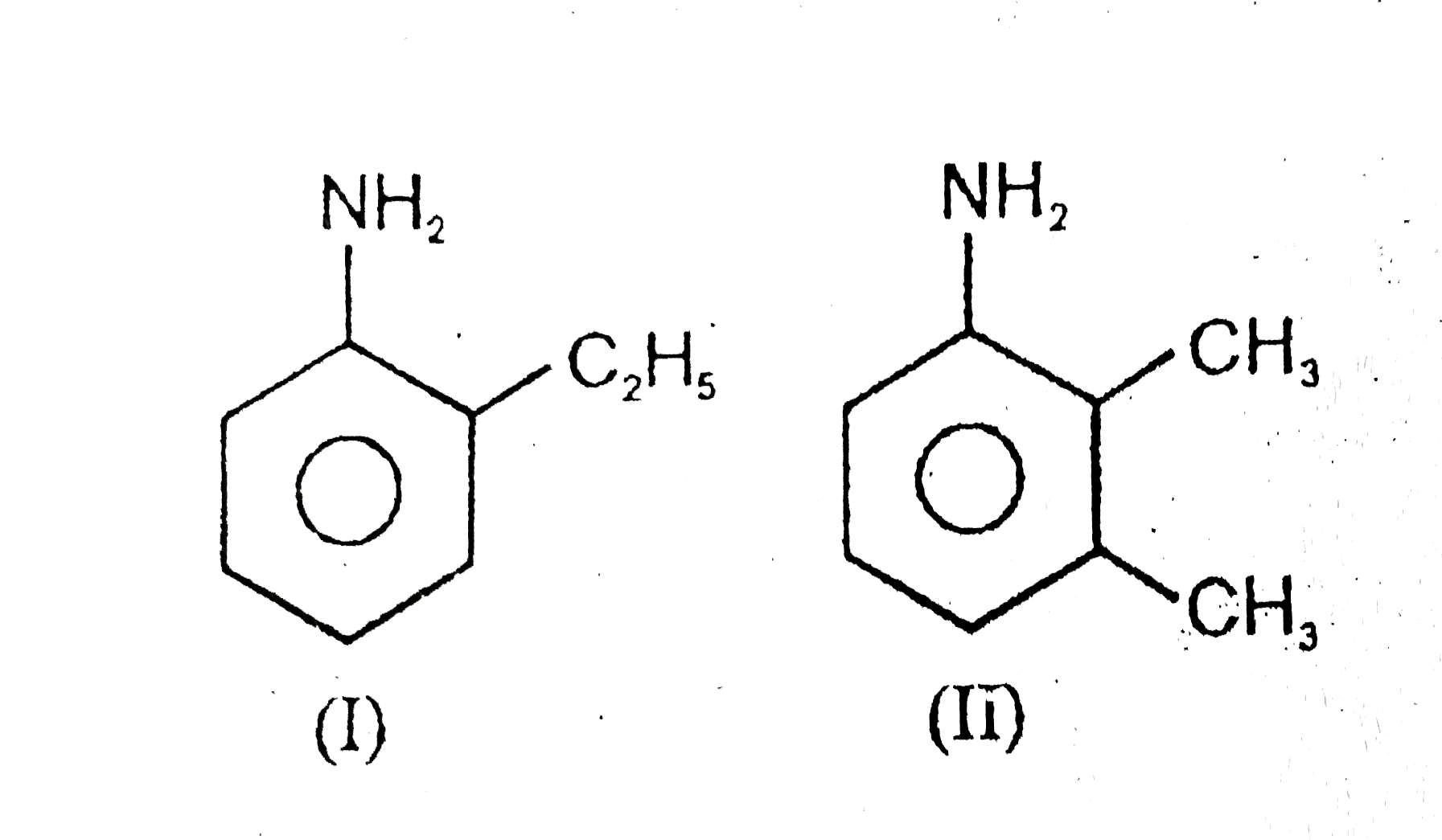
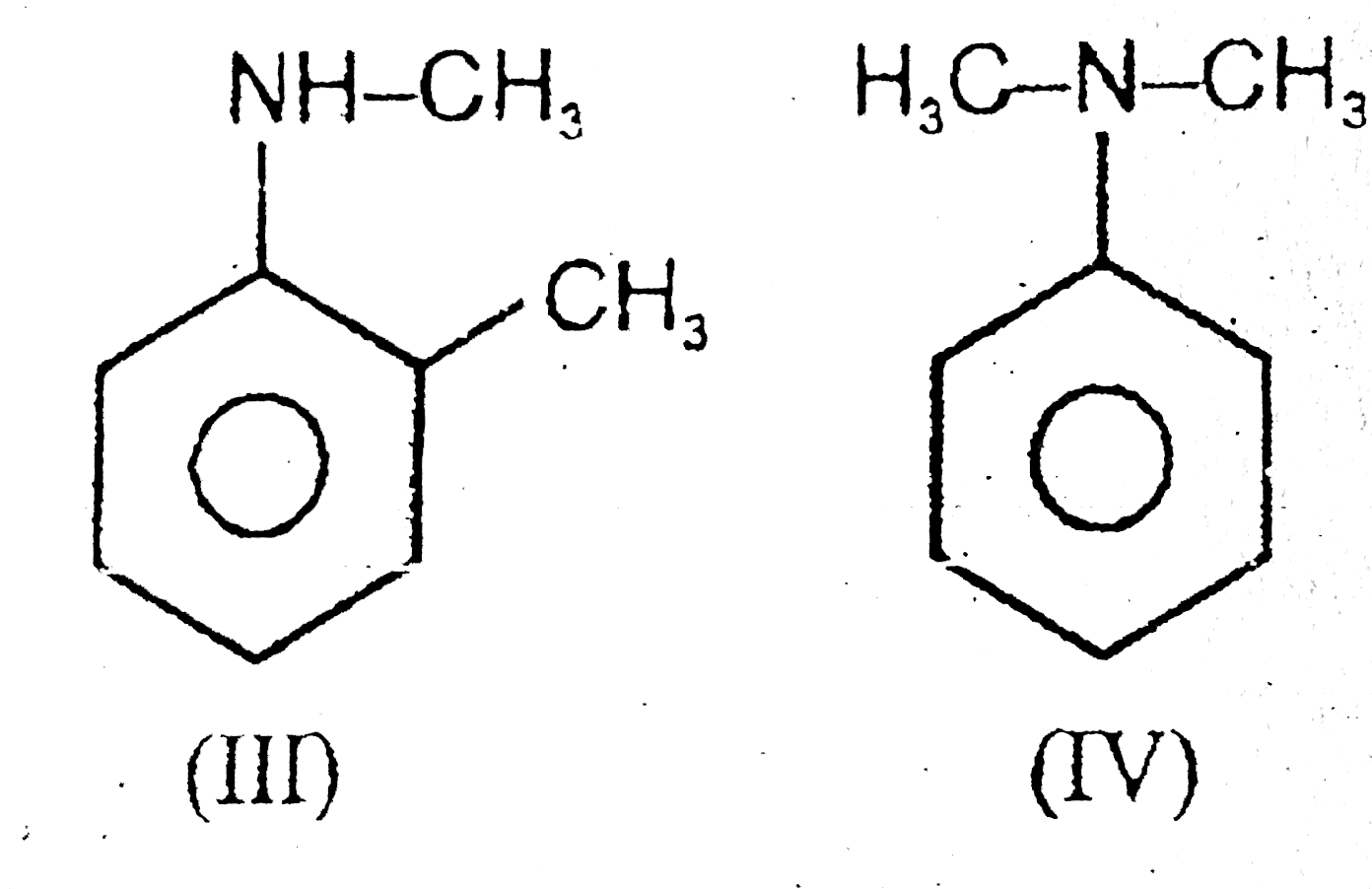
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- (A) I & II are chain isomers (B) I & III are functional isomers ...
Text Solution
|
- Amino acids are functional isomers of (i) oximes (ii) amides (iii) alk...
Text Solution
|
- (A) I & II are chain isomers (B) I & III are functional isomers ...
Text Solution
|
- Match the column- {:("Column I","Column II"),("A Toxins","i. Morphin...
Text Solution
|
- (i) Write one chain isomer of 1-methoxy-2-methyl propane. (ii) Write o...
Text Solution
|
- I) (A) and (B) are diastereomers II) (B) and (D) are enantiomers III) ...
Text Solution
|
- Match the followings and choose the correct answer. (A) (a - ii), (b -...
Text Solution
|
- Match the following with reference to cockroach and choose the correct...
Text Solution
|
- Match the followings and choose the correct answer. (A) (a - iii)...
Text Solution
|