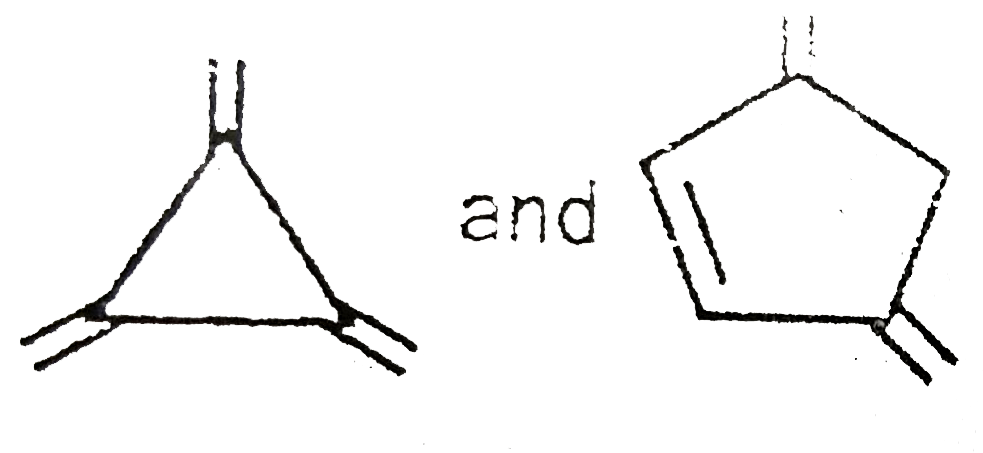
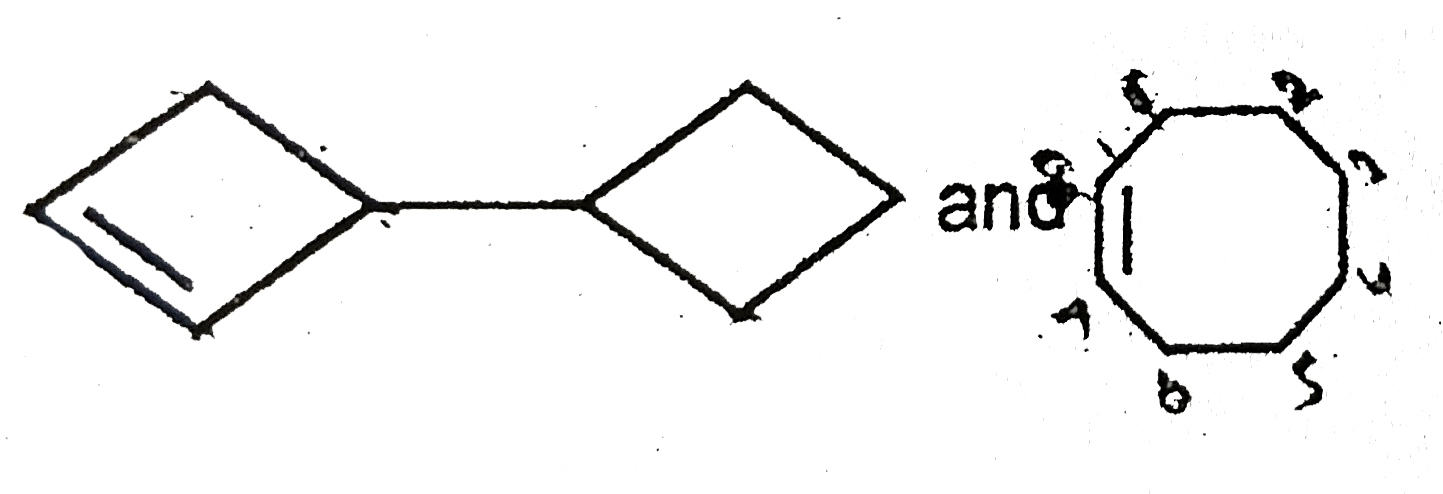
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In which of the following pair of compounds having same general formul...
Text Solution
|
- Which of the following pairs of physical quantites does not have same ...
Text Solution
|
- Which of the following pairs of componds have the same general formula...
Text Solution
|
- Which of the following pairs have the same general molecular formula C...
Text Solution
|
- In which of the following pair of compounds having same general formul...
Text Solution
|
- The pair of compounds having the same general formula.
Text Solution
|
- STATEMENT -1 : Compound having same general formula may have differen...
Text Solution
|
- Which of the following pair have same dimensional formula?
Text Solution
|
- Which of the following pairs of physical quantities does not have same...
Text Solution
|