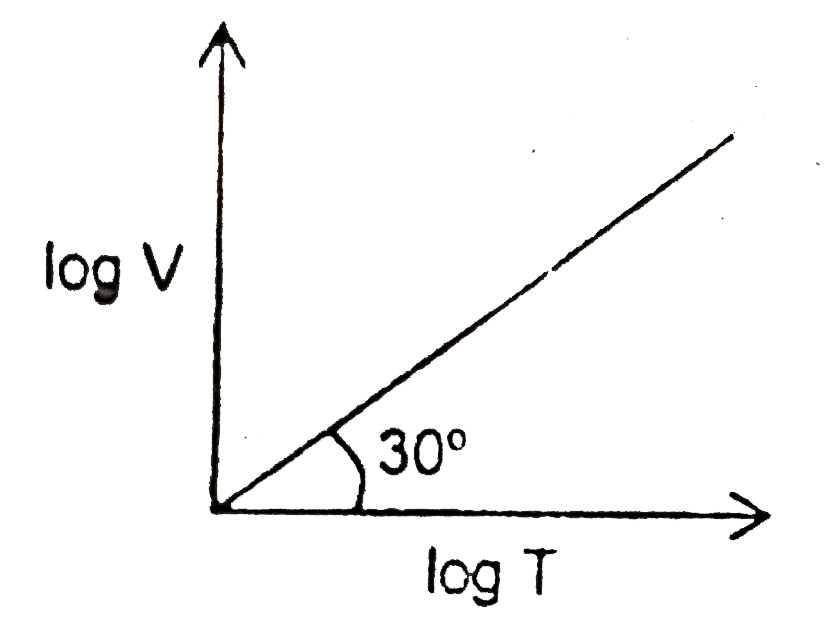
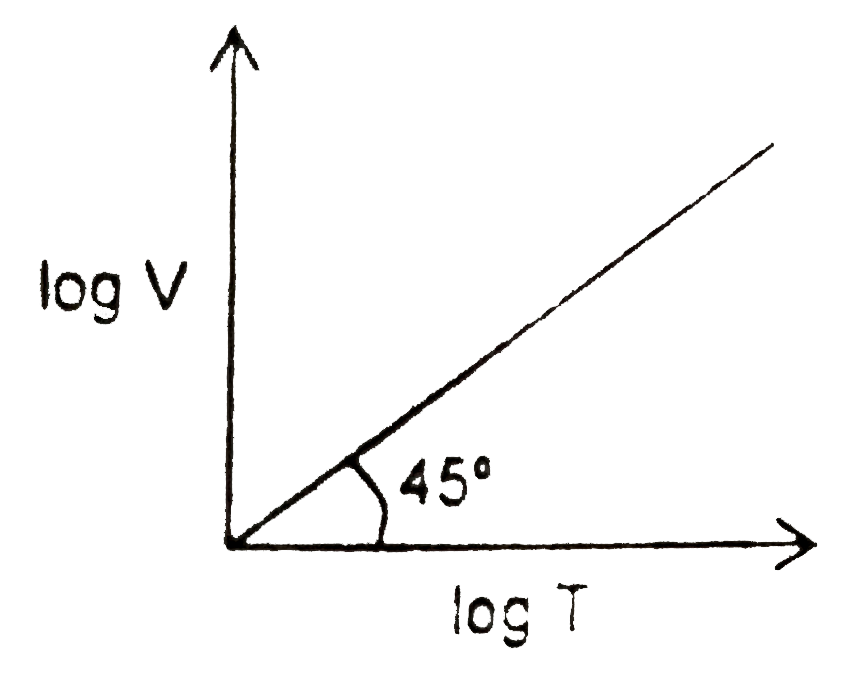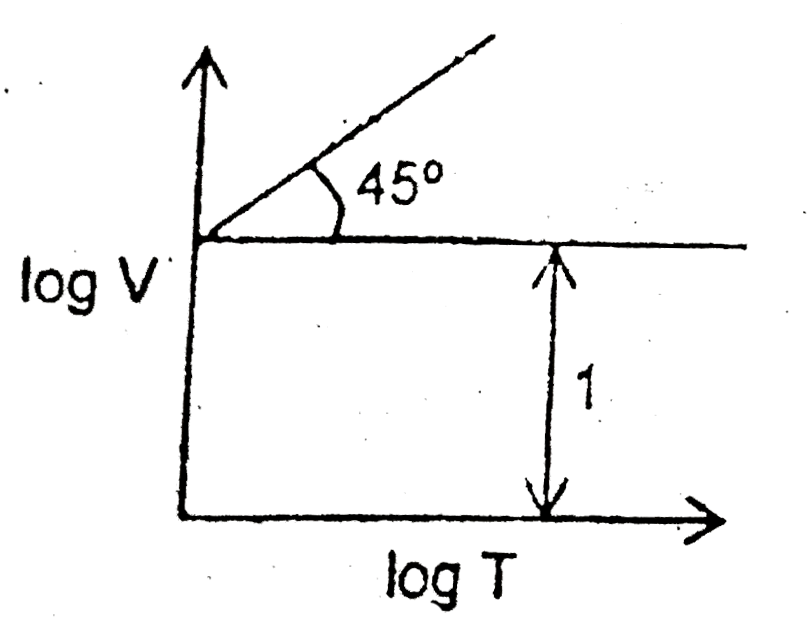A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For a closed container containing 100 mole of an ideal gas fitted with...
Text Solution
|
- The intercept of the line drawm for log P (P in atm) and log (1)/(V) (...
Text Solution
|
- For a closed container containing 100 mol of an ideal gas fitted with ...
Text Solution
|
- Identify the correct statement when a fixed amount of ideal gas is hea...
Text Solution
|
- For a closed container containing 10 moles of an ideal gas, at constan...
Text Solution
|
- 10 moles of an ideal gas is subjected to an isochoric process (volume ...
Text Solution
|
- Log P vs log V curve is plotted for an ideal gas, which is true for ...
Text Solution
|
- For an ideal gas, under isobaric condition, a graph between log V vs l...
Text Solution
|
- Ten moles of an ideal gas are filled in a closed vessel. The vessel ha...
Text Solution
|