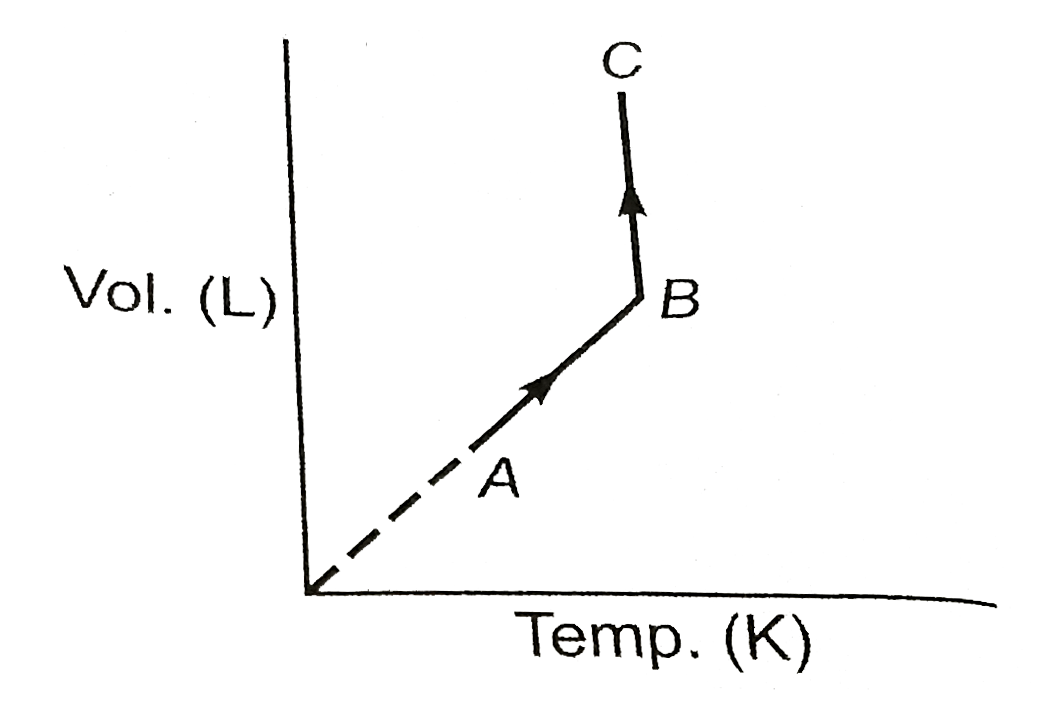Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Two moles of a triatomic linear gas (neglect vibration degree of freed...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process start...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process starti...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process start...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process starti...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process start...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process starti...
Text Solution
|
- Two moles of triatomic linear gas are taken through a reversible proce...
Text Solution
|
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|