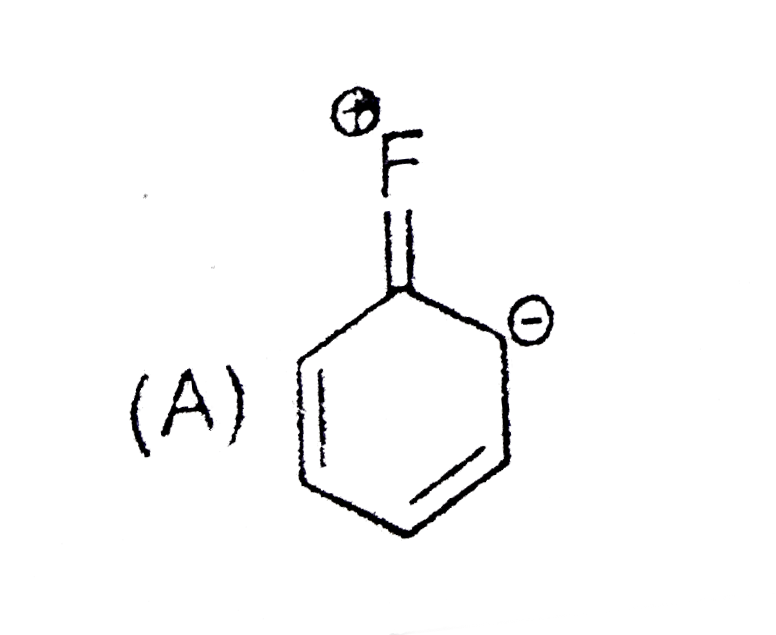
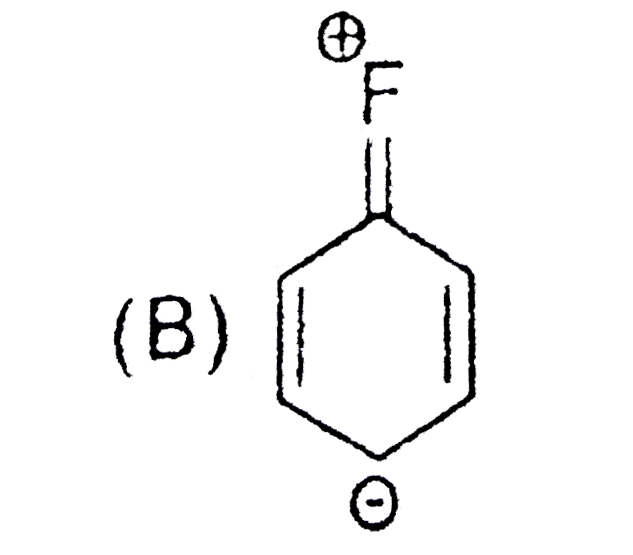
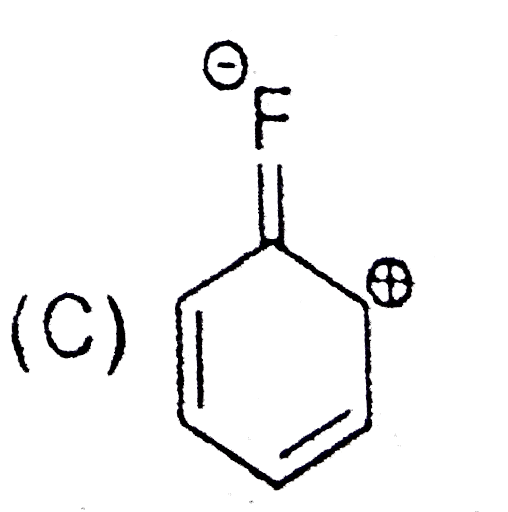
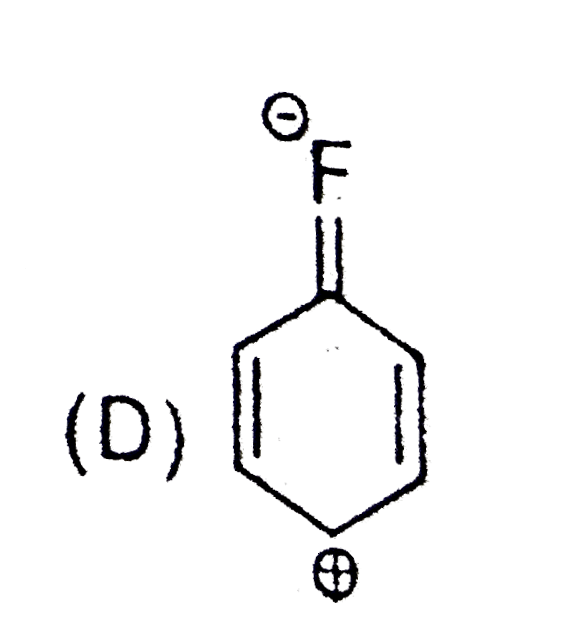A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
TEST PAPERS
RESONANCE ENGLISH|Exercise Chemistry|701 VideosTEST PAPERS
RESONANCE ENGLISH|Exercise PT-01|30 VideosTEST PAPERS
RESONANCE ENGLISH|Exercise PART - III CHEMISTRY|46 VideosSURFACE CHEMISTRY
RESONANCE ENGLISH|Exercise Section - 5|1 VideosTEST SERIES
RESONANCE ENGLISH|Exercise CHEMISTRY|50 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST PAPERS-PART - III CHEMISTRY
- 16 g of oxygen and 4 g of hydrogen are placed in 1.12 litre flask at 0...
Text Solution
|
- Calculate the no. of mole in 150 g of CaCO3
Text Solution
|
- Calculate the no. of mole in 100 g of CaCO3
Text Solution
|
- The number of atoms in 20gm of CaCO3 is:
Text Solution
|
- 32 g of oxygen and 1 g of hydrogen are placed in 1.12 litre flask at 0...
Text Solution
|
- The number of atoms in 100gm of CaCO3 is:
Text Solution
|
- The number of atoms in 50gm of CaCO3 is:
Text Solution
|
- A liquid confined inside an adiabatic is suddenly taken from state 1 t...
Text Solution
|
- 1 mol of an idel gas is allowed to expand isothermally at 27^(@)C til...
Text Solution
|
- 32 g of oxygen and 2 g of hydrogen are placed in 1.12 litre flask at 0...
Text Solution
|
- Find the temperature at which 3 moles of SO2 will occupy a volume of 1...
Text Solution
|
- Calculate the pressure exerted by one mole CO2 of gas at 273 K, if th...
Text Solution
|
- The impossible resonating structures of fluorobenzene is/are :
Text Solution
|
- Find out correct statement/s about squaric acid diansion:
Text Solution
|
- Which of the following groups exerts +m effect when attached with benz...
Text Solution
|
- Among the following reaction, which from salicylic acid.
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- From the compound shown below, choose which is/are aromatic.
Text Solution
|
- Which of the following will have zero dipole moment?
Text Solution
|
- 4.8 g of oxygen and 3.4 g of hydrogen are placed in 1.12 litre flask a...
Text Solution
|