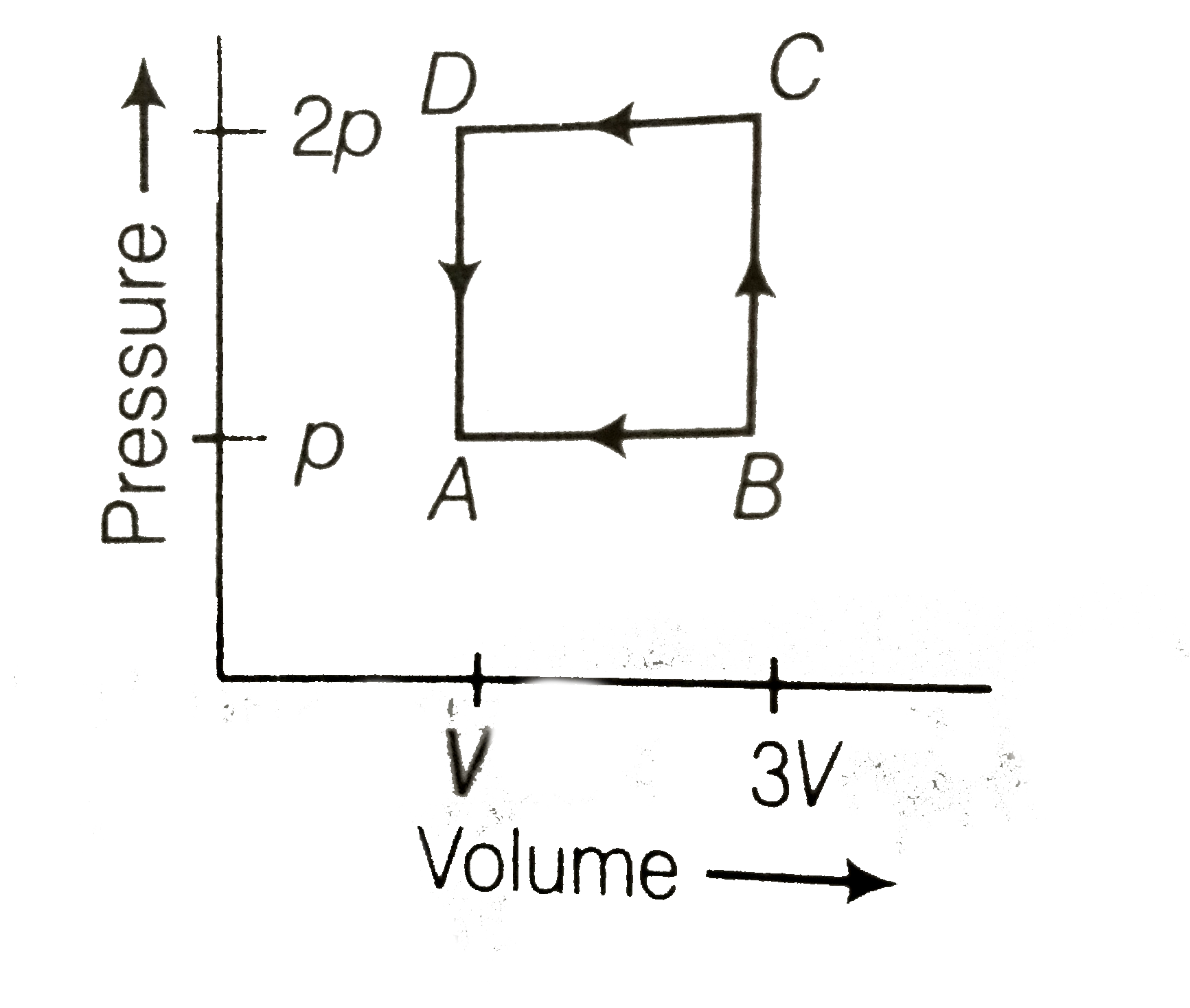A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-KINETIC THEORY OF GASES AND THERMODYNAMICS-Exercise
- The value of gamma of linear, arragement of triatomic gas molecules is
Text Solution
|
- If the molar specific heat of a gas at constant pressure is 7/2R, then...
Text Solution
|
- The internal energy change in a system that has absorbed 2 kcal of hea...
Text Solution
|
- If Delta U and Delta W represent the increase in internal energy and w...
Text Solution
|
- A monoatomic gas at pressure P(1) and volume V(1) is compressed adiaba...
Text Solution
|
- A mass of diatomic gas (gamma = 1.4) at a pressure of 2 atmosphere is ...
Text Solution
|
- When temperature of a gas is increased then which of the following sta...
Text Solution
|
- A thermos flask contains coffee. It is vigorously sheken, considering ...
Text Solution
|
- The work done by a gas taken through the closed process ABCA, see fig...
Text Solution
|
- A thermodynamic system is taken through the cycle ABCD as shown in fig...
Text Solution
|
- For adiabatic process of an ideal gas the value of (dP)/P is equals to
Text Solution
|
- The change in internal energy of two moles of a gas during adiabatic e...
Text Solution
|
- isobaric modulus of elasticity is
Text Solution
|
- The internal energy of one mole mono-atomic gas is
Text Solution
|
- A carnot's engine works between a source at a temperature of 27^(@)C a...
Text Solution
|
- If the ratio of specific heat of a gas at constant pressure to that at...
Text Solution
|
- In an adiabatic process-
Text Solution
|
- The amount of heat given to a system in a cyclic thermodynamical proce...
Text Solution
|
- There are two parts of a vessel. The pressure in one part is P and its...
Text Solution
|
- Statement-I: it is possible for both the pressure and volume of a mono...
Text Solution
|