A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-PART TEST 6-Exercise
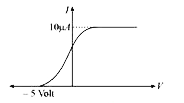
- The graph between photo electric current and cathode potential when th...
Text Solution
|
- For a pure semiconductor energy band shown at 0K choose correct option...
Text Solution
|
- Photo electric effect can be explained only by assuming that light A...
Text Solution
|
- Light of two different frequencies whose photons have energies 1 eV an...
Text Solution
|
- In an interference pattern the (n+4)^(th) blue bright fringe and n^(th...
Text Solution
|
- A particle of mass m is projected form ground with velocity u making a...
Text Solution
|
- When a metallic surface is illuminated with monochromatic light of wav...
Text Solution
|
- Light of wavelength 400 nm is incident continuously on a caesium ball ...
Text Solution
|
- The Rutherford alpha-particle experiment shown that most of the alpha-...
Text Solution
|
- Which one of the following graphs in figure shows the variation of pho...
Text Solution
|
- Two electrons starting from rest are accelerated by equal potential di...
Text Solution
|
- Let mp be the mass of a proton , mn the mass of a neutron, M1 the mass...
Text Solution
|
- In the photoelectric experiment, if we use a monochromatic light, the ...
Text Solution
|
- In a photoelectric effect experiment, stopping potential changes by 30...
Text Solution
|
- The photon radiated from hydrogen corresponding to 2^(nd) line of Lyma...
Text Solution
|
- An electron of mass m when accelerated through a potential difference ...
Text Solution
|
- A photon of energy 12.09 eV is absorbed by an electron in ground state...
Text Solution
|
- What percentage increase in wavelength leads to 75% loss of photon ene...
Text Solution
|
- Statement 1: Though light of a single frequency (monochromatic light) ...
Text Solution
|
- Staements I: .zX^A undergoes 2 alpha-decays, 2 beta-decays (negative b...
Text Solution
|