A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
RESONANCE ENGLISH|Exercise Advanved level problems|17 VideosATOMIC PHYSICS
RESONANCE ENGLISH|Exercise Exercise-2 Part-III : Comprehension|12 VideosALTERNATING CURRENT
RESONANCE ENGLISH|Exercise HIGH LEVEL PROBLEMS|11 VideosCAPACITANCE
RESONANCE ENGLISH|Exercise High Level Problems|16 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ATOMIC PHYSICS-Exercise -3 part -I JEE (Advanced)
- In a mixture of H-He^(+) gas (He^(+) is singly ionized He atom), H ato...
Text Solution
|
- In a mixture of H-He^(+) gas (He^(+) is singly ionized He atom), H ato...
Text Solution
|
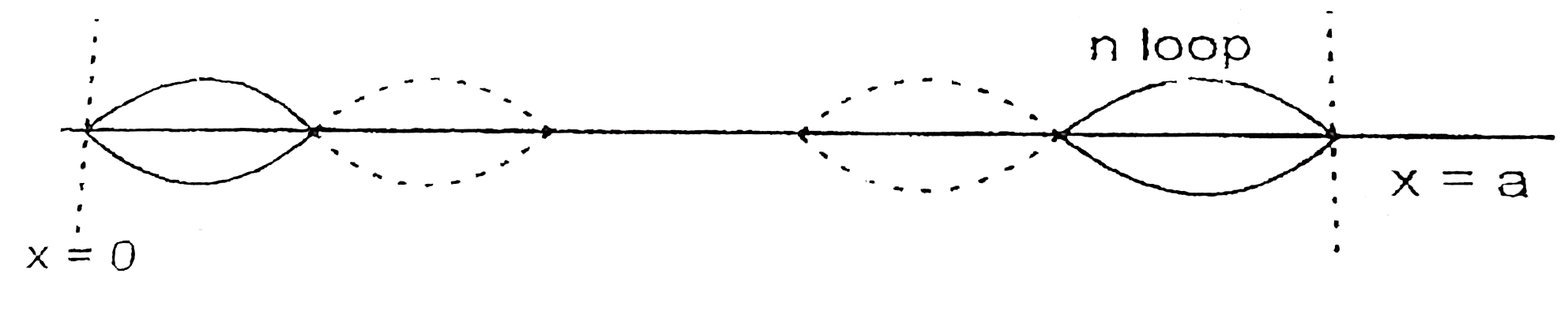
- When a particle is restricted to move along x-axis between x=0 and x=a...
Text Solution
|
- When a particle is restricted to move along x-axis between x=0 and x=a...
Text Solution
|
- When a particle is restricted to move along x-axis between x=0 and x=a...
Text Solution
|
- Photoelectric effect experiments are performed using three different m...
Text Solution
|
- An alpha particle and a proton are accelerated from rest by a potentia...
Text Solution
|
- The key feature of Bohr's spectrum of hydrogen atom is the quantizatio...
Text Solution
|
- The key feature of Bohr's theory of spectrum of hydrogen atom is the q...
Text Solution
|
- The key feature of Bohr's theory of spectrum of hydrogen atom is the q...
Text Solution
|
- if the wavelength of the first line of the balmer series of hydrogen i...
Text Solution
|
- A dence collection of equal number of electrona and positive ions is ...
Text Solution
|
- A dence collection of equal number of electrona and positive ions is ...
Text Solution
|
- A silver sphere of radius 1 cm and work function 4.7 eV is suspended f...
Text Solution
|
- A pulse of light of duration 100 ns is absorbed completely by a small ...
Text Solution
|
- The work function of Silver and sodium are 4.6 and 2.3 eV, respective...
Text Solution
|
- The radius of the orbit of an electron in Hydrogen-like aton is 4.5 al...
Text Solution
|
- if lambda(Cu) is the wavelength of Kalpha, X-ray line fo copper (atomi...
Text Solution
|
- A metal surface is illuminated by light of two different wavelengths 2...
Text Solution
|
- Consider a hydrogen atom with its electron in the n^(th) orbital An el...
Text Solution
|