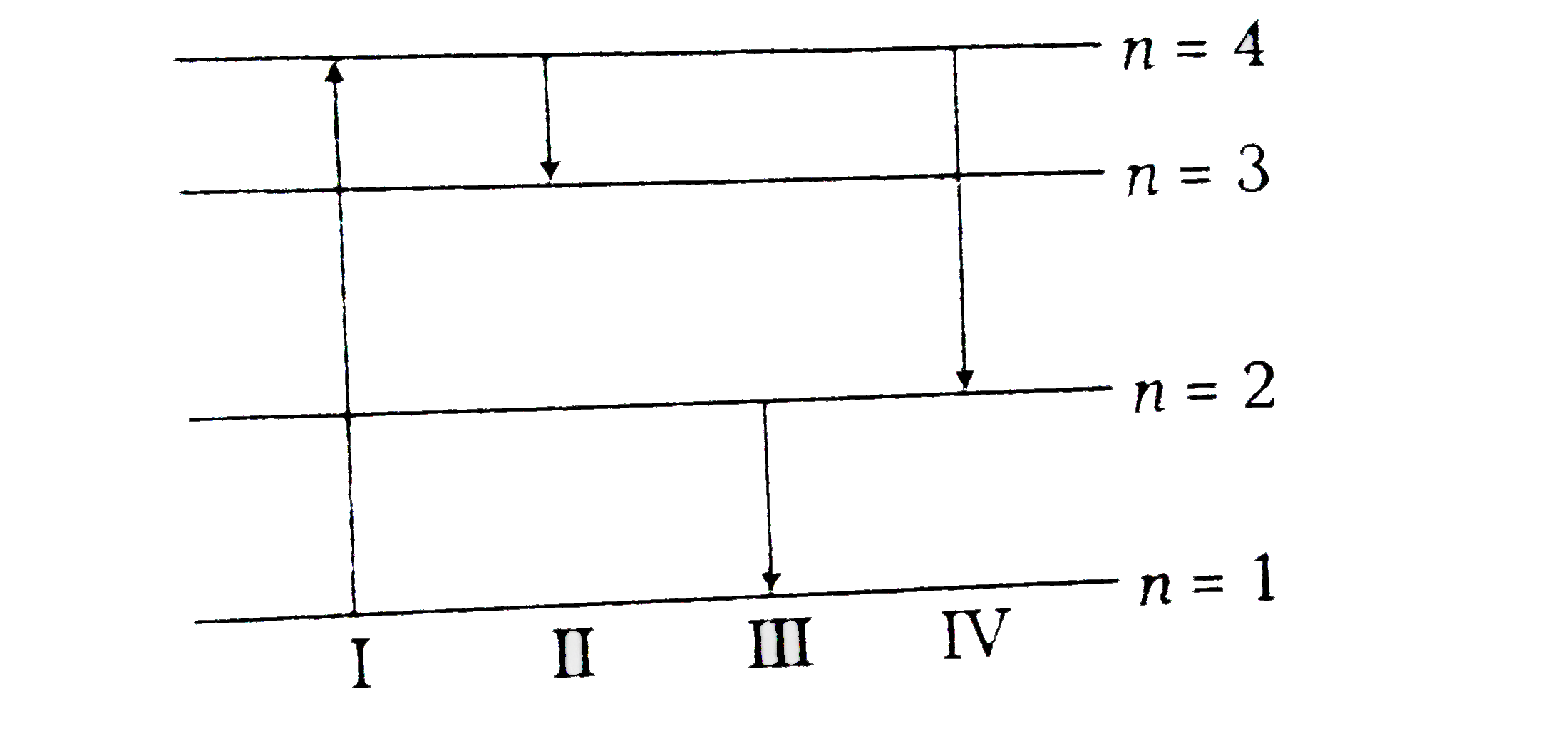A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
RESONANCE ENGLISH|Exercise Advanved level problems|17 VideosATOMIC PHYSICS
RESONANCE ENGLISH|Exercise Exercise-2 Part-III : Comprehension|12 VideosALTERNATING CURRENT
RESONANCE ENGLISH|Exercise HIGH LEVEL PROBLEMS|11 VideosCAPACITANCE
RESONANCE ENGLISH|Exercise High Level Problems|16 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ATOMIC PHYSICS-Exercise -3 part -I JEE (Advanced)
- The intensity of gamma radiation from a given source is I. on passing ...
Text Solution
|
- A photo cell is illuminated by a small bright source placed 1m away Wh...
Text Solution
|
- The diagram shows the energy levels for an electron in a certain atom....
Text Solution
|
- If the kinetic energy of a free electron doubles . Find the factor by ...
Text Solution
|
- The time taken by a photoelectron to come out after the photon strikes...
Text Solution
|
- An alpha nucleus of energy (1)/(2)mv^(2) bomobards a heavy nuclear tar...
Text Solution
|
- The threshold frequency for a metallic surface corresponds to an energ...
Text Solution
|
- The anode voltage of a photocell is kept fixed. The wavelength lamda o...
Text Solution
|
- Photon of frequency v has a momentum associated with it. If c is the v...
Text Solution
|
- Which of the following transitions gives photon of maximum energy?
Text Solution
|
- suppose an electron is attracted towards the origin by a force k//r, w...
Text Solution
|
- The transition from the state n = 4 " to " n = 3 in a hydrogen like at...
Text Solution
|
- The surface of a metal is illuminated with the light of 400nm. The kin...
Text Solution
|
- Statement-1 : When ultraviolet light is incidient on a photocell, its ...
Text Solution
|
- If a source of power 4 kW produces 10^(20) photons/second, the radiati...
Text Solution
|
- Energy required for the electron excitation in Li^(++) from the first ...
Text Solution
|
- This question has statement - 1 and statement - 2 of the four choice g...
Text Solution
|
- After absobing a slowly moving neutron of mass m(N) (mometum ~0), a nu...
Text Solution
|
- Hydrogen atom is excited from ground state to another state with prin...
Text Solution
|
- A diatomic molecule is made of two masses m(1) and m(2) which are sepa...
Text Solution
|