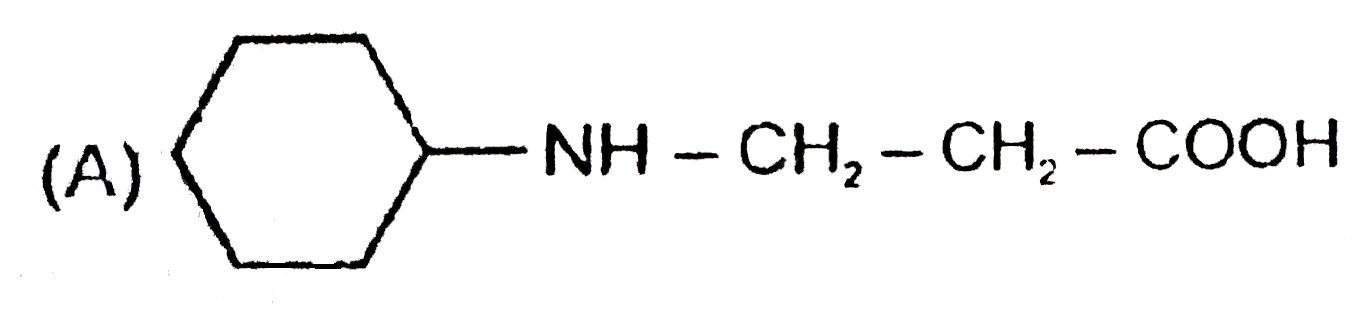
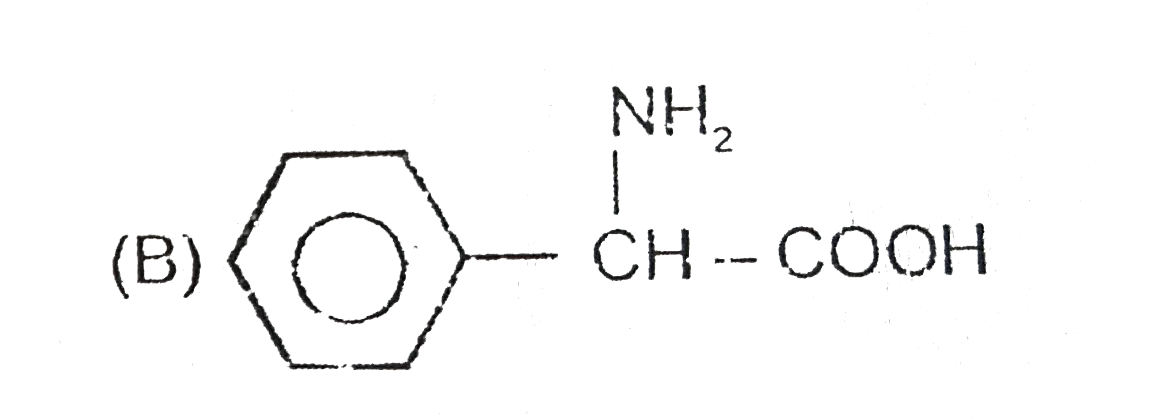
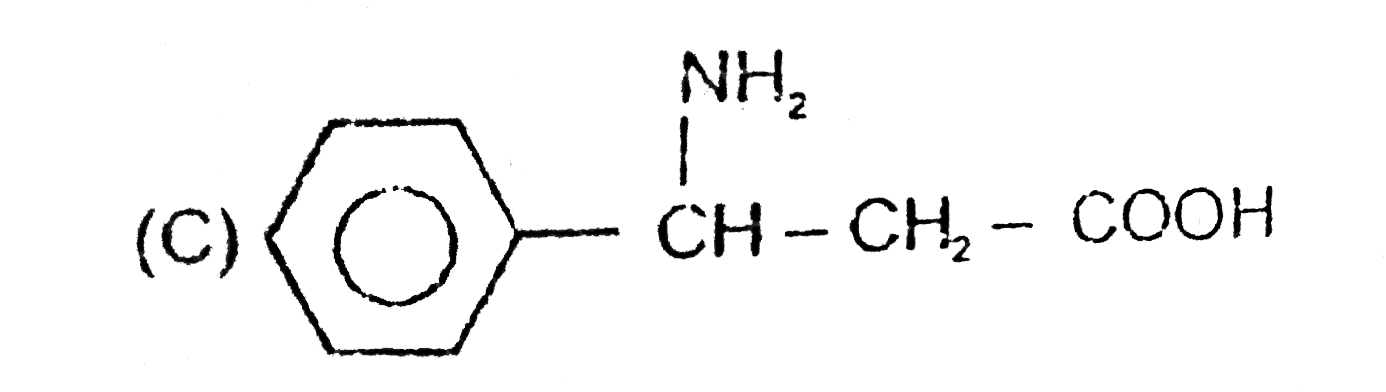
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES
RESONANCE ENGLISH|Exercise Ex-1(sec-C)Part-II|8 VideosBIOMOLECULES
RESONANCE ENGLISH|Exercise Ex-1(sec-D)Part-II|10 VideosBIOMOLECULES
RESONANCE ENGLISH|Exercise Ex-1(sec-A)Part-II|17 VideosBASIC CONCEPTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosBIOMOLECULES & POLYMER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|34 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-BIOMOLECULES-Ex-1(sec-B)Part-II
- Which of the following alpha-amino acids is not optically active?
Text Solution
|
- 4 L of gas is enclosed in a vessel at a pressure of 760 mm Hg. If temp...
Text Solution
|
- The force of attraction between the neighbouring peptide chains is
Text Solution
|
- Which one of the following is a basic amino acid ?
Text Solution
|
- Which of the following is alpha-"amino" acid ?
Text Solution
|