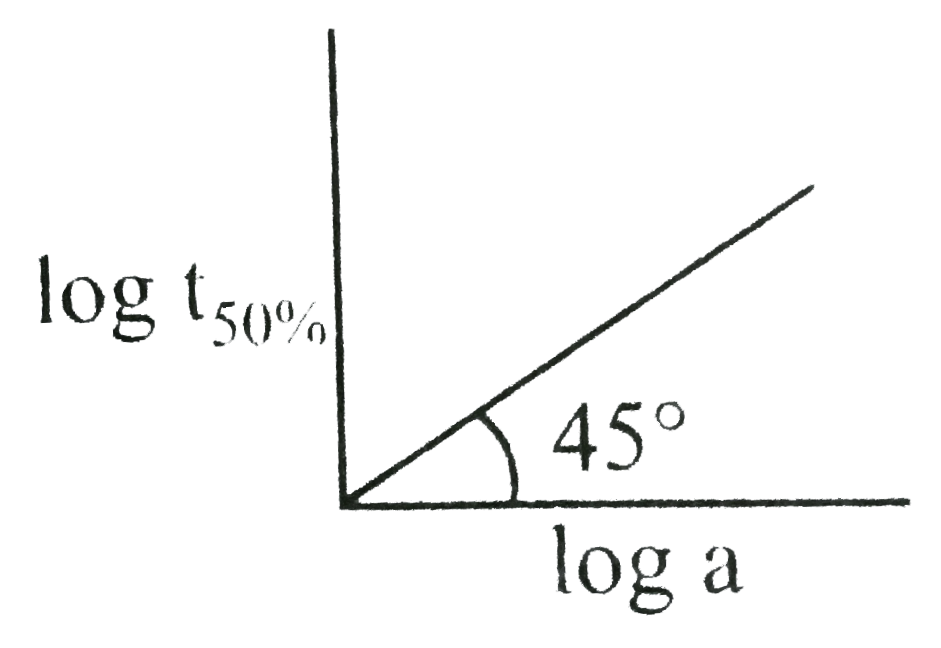A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CHEMICAL KINETICS-PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)
- For a chemical reaction A rarr Products, the rate of disappearance of ...
Text Solution
|
- At the point of intersection of the two curves shown, the conc. of B i...
Text Solution
|
- The decomposition of N(2)O(5) occurs as, 2N(2)O(5) rarr4NO(2)+O(2) and...
Text Solution
|
- The rate constant for an isomerization reaction, A rarr B is 4.5 xx 10...
Text Solution
|
- A hypothetical reaction A(2) + B(2) rarr 2AB follows the mechanism as ...
Text Solution
|
- For an exothermic chemical process ocuuring in two process occuring in...
Text Solution
|
- For the reaction , 2NO2+F5rarr 2NO2F following mechanism has been pro...
Text Solution
|
- For the reaction, 2NO(g) + 2H(2)(g) rarr N(2)(g) + 2H(2)O(g) The ...
Text Solution
|
- What will be the order of reaction and rate constant for a chemical ch...
Text Solution
|
- The unit of rate constant of elementary reaction depends upon the:
Text Solution
|
- For an elementary reaction 2A+BtoA(2)B if the volume of vessel is quic...
Text Solution
|
- The decomposition of azo methane, at certain temperature according to ...
Text Solution
|
- For a complex reaction A overset(k) rarr products E(a1)=180kJ //mol ...
Text Solution
|
- Atoms .(7)^(A)X, (8)^(B)Y " and " (9)^(17)Z are such that .(8)Y is an ...
Text Solution
|
- The number of neutrons accompanying the formation of .(54)Xe^(139) and...
Text Solution
|
- A sample of radiative substance is found 90% of it’s initial amount af...
Text Solution
|
- For a given reaction A rarr Product, rate is 1xx10^(-4)M s^(-1) when ...
Text Solution
|
- Milk turns sour at 40^(@)C three times as faster as 0^(@)C. Hence E(a)...
Text Solution
|
- Graph between log k and 1//T [k rate constant (s^(-1)) and T and the t...
Text Solution
|
- In a first order reaction, the concentrations of the reactant, 30 minu...
Text Solution
|