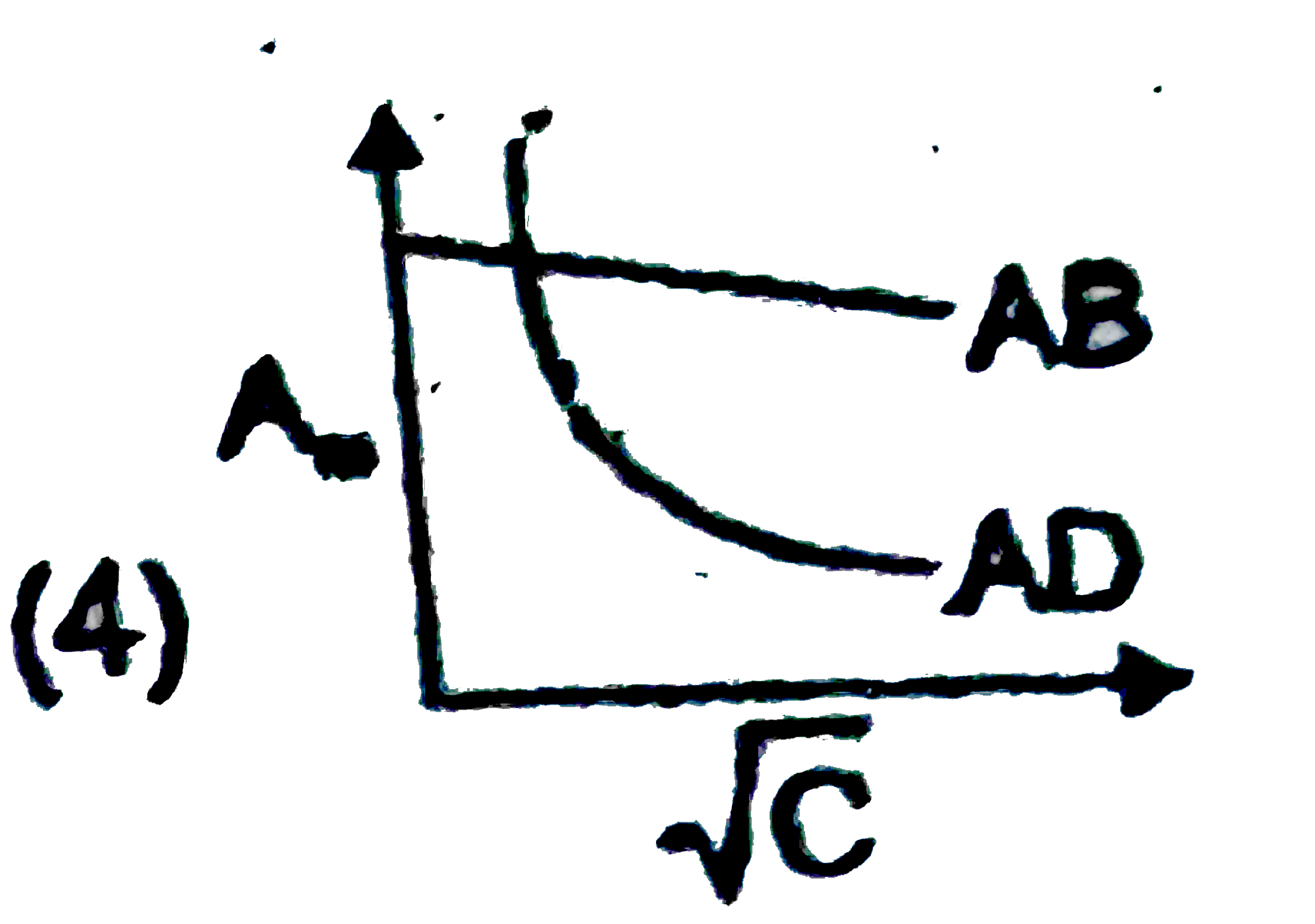A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ELECTRO CHEMISTRY-PHYSICAL CHEMITRY (ELECTROCHEMISTRY)
- E(H^(+)//H(2))^(0)=0.00V, then E(D^(+)//D(2))^(0) at 25^(@)C will be
Text Solution
|
- When an electric current is passed through acidified water, 112 mL of ...
Text Solution
|
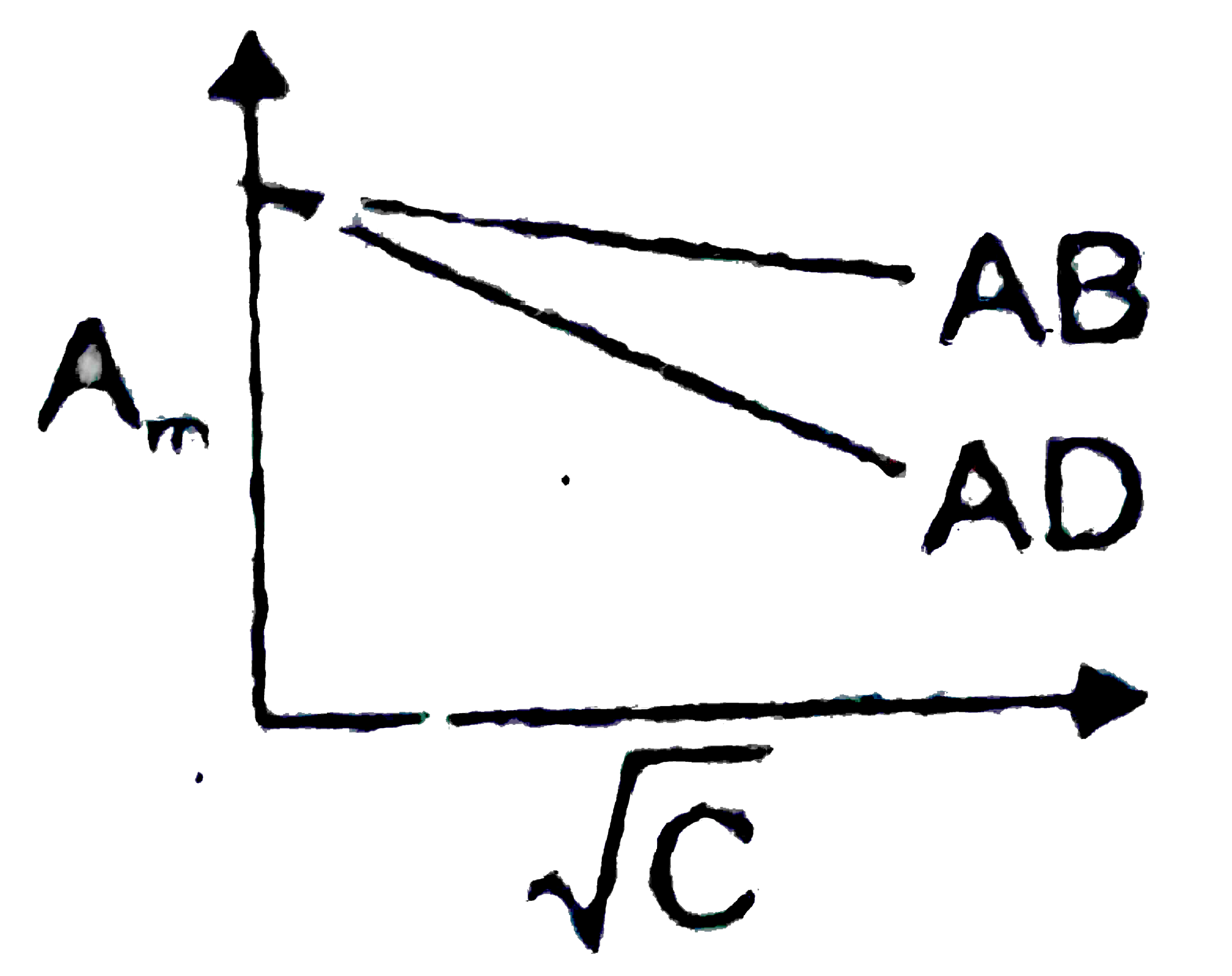
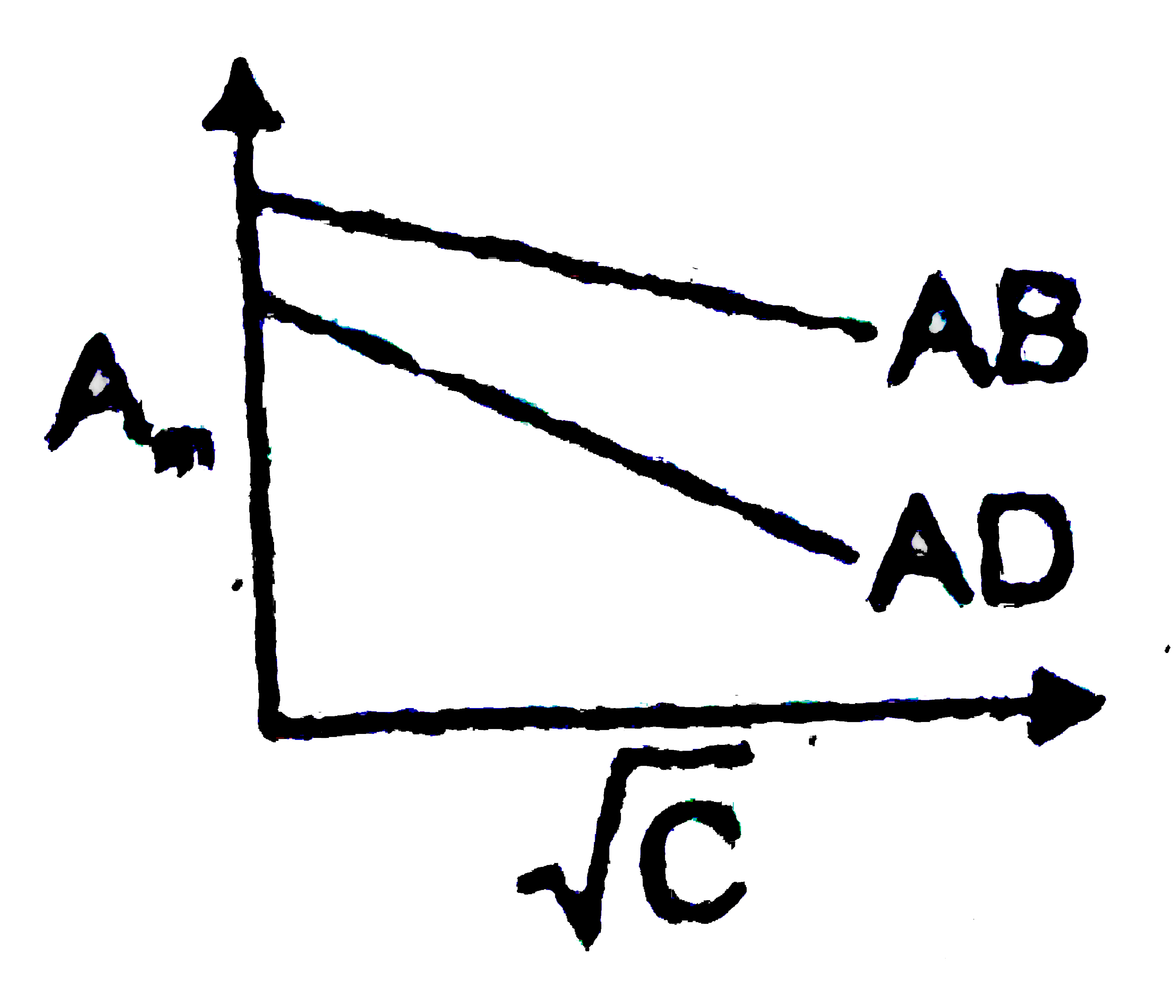
- Which of the following graphs are correct for strong electrolyte AD an...
Text Solution
|
- Number of electrons lost during electrolysis of 0.14g of N^(-3) is -(N...
Text Solution
|
- Aqueous solution of NaCl containing a small amount of MeOH ( methyl o...
Text Solution
|
- The spontaneous redox reaction//s among the follow is// are (a) 2Fe...
Text Solution
|
- During discharging of lead-storage acid battery following reaction tak...
Text Solution
|
- 0.169 gram of copper is deposited on the cathode by a current of 32 mi...
Text Solution
|
- Using the standard electrode potential values given below, decide whi...
Text Solution
|
- For the half cell At pH=2. Electrode potential is :
Text Solution
|
- In the electrolysis of an aqueous potassium sulphate solution, the p...
Text Solution
|
- How many electrons are there in one coulomb electricity?
Text Solution
|
- Electrolysis can be used to determine atomic masses. A current of 0.55...
Text Solution
|
- Calculate the current (in mA) required to deposite 0.195g of platinum ...
Text Solution
|
- An aqueous solution containing 1 M each of Au^(3+),Cu^(2+),Ag^+,Li^+ i...
Text Solution
|
- Based on the following information arrange four metals A,B,C and D in ...
Text Solution
|
- The standard electrode potential for the following reaction is +1.33 V...
Text Solution
|
- Ag|AgCl|Cl^(-)(C(2))||Cl^(-)(C(1))|AgCl|Ag for this cell DeltaG is neg...
Text Solution
|
- Resistance of a decimolar solution between two electrodes 0.02 meter a...
Text Solution
|
- Equivalent conductivity of Fe2(SO4)3 is related ot molar conductivity ...
Text Solution
|