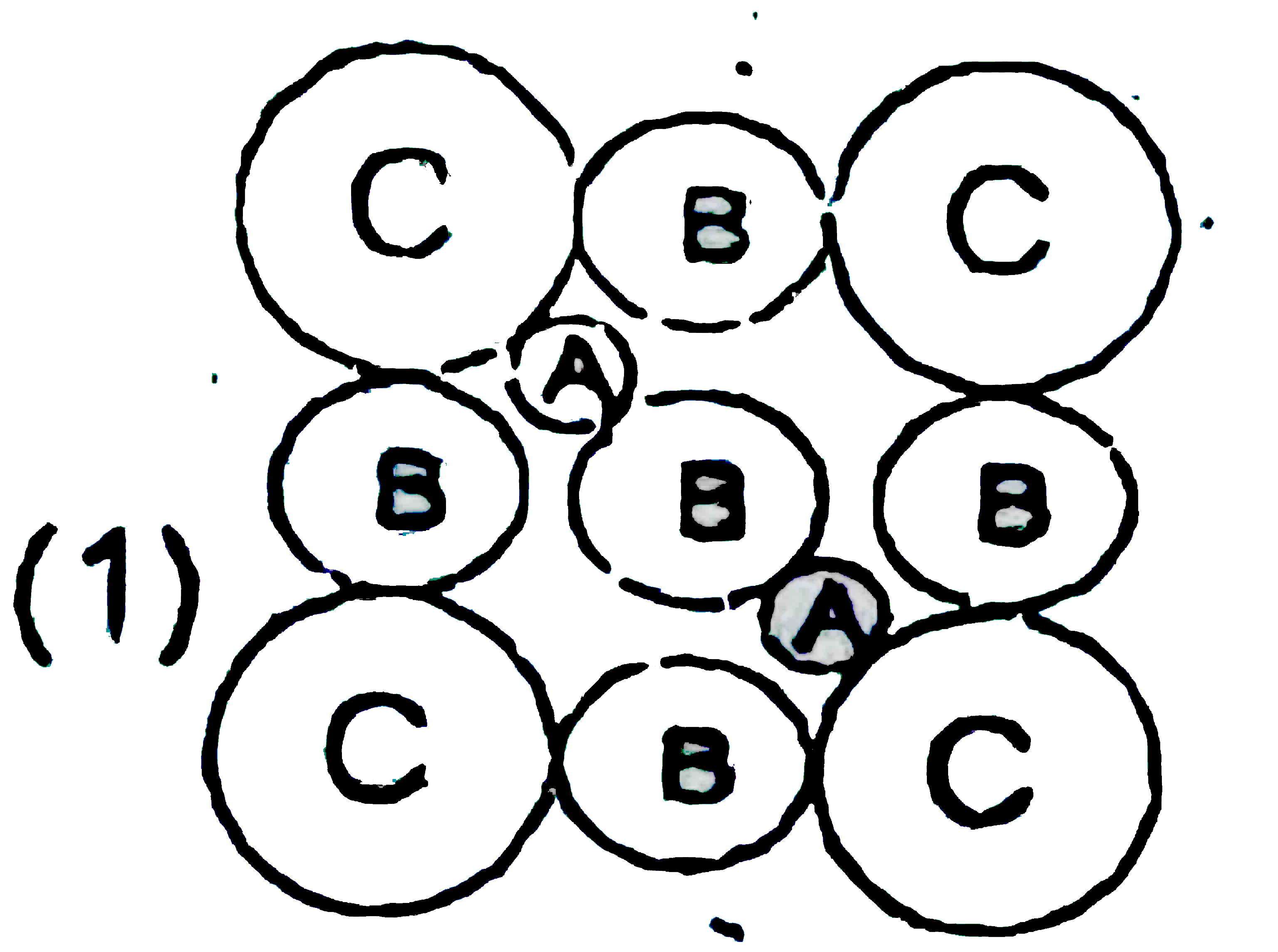
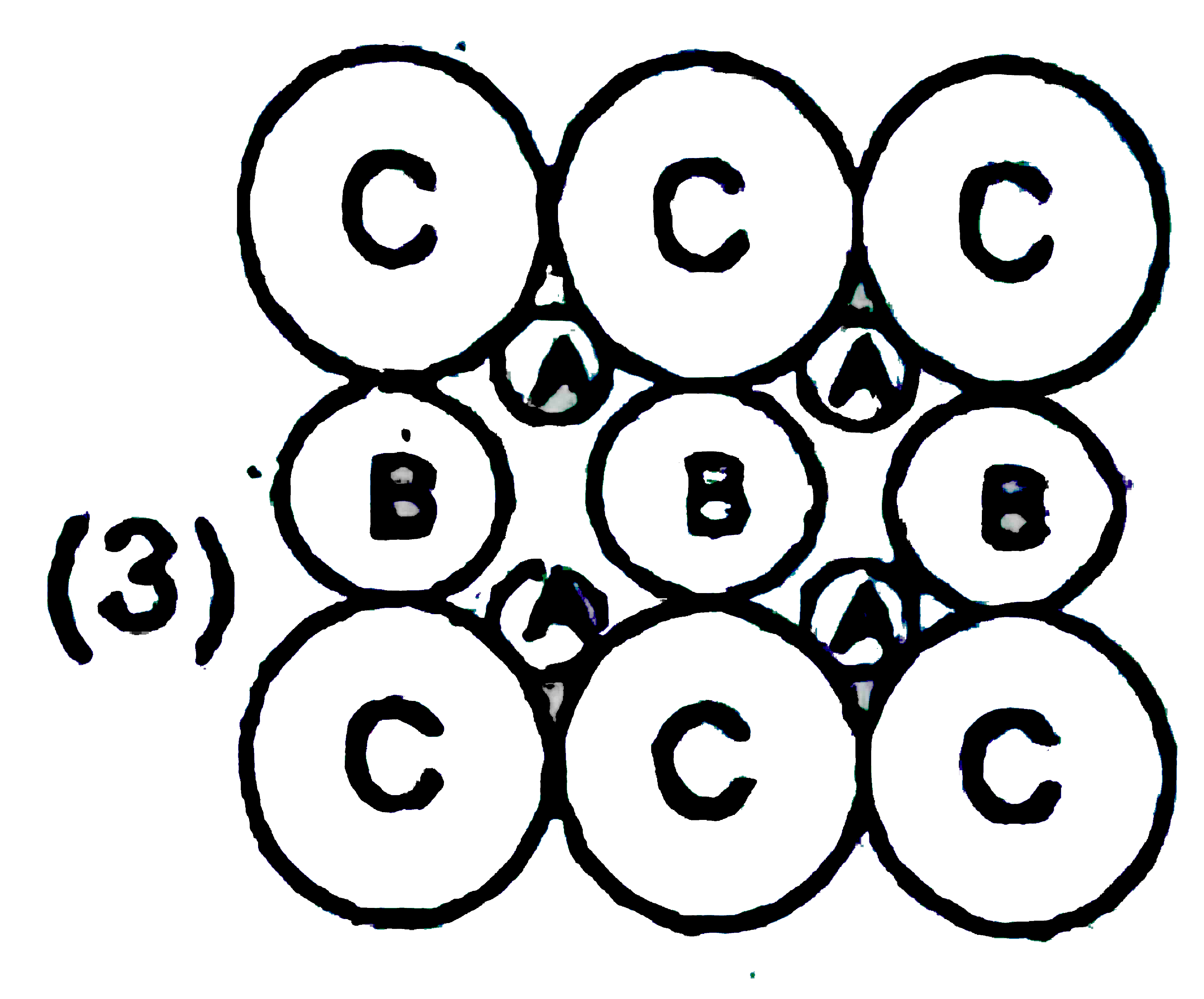
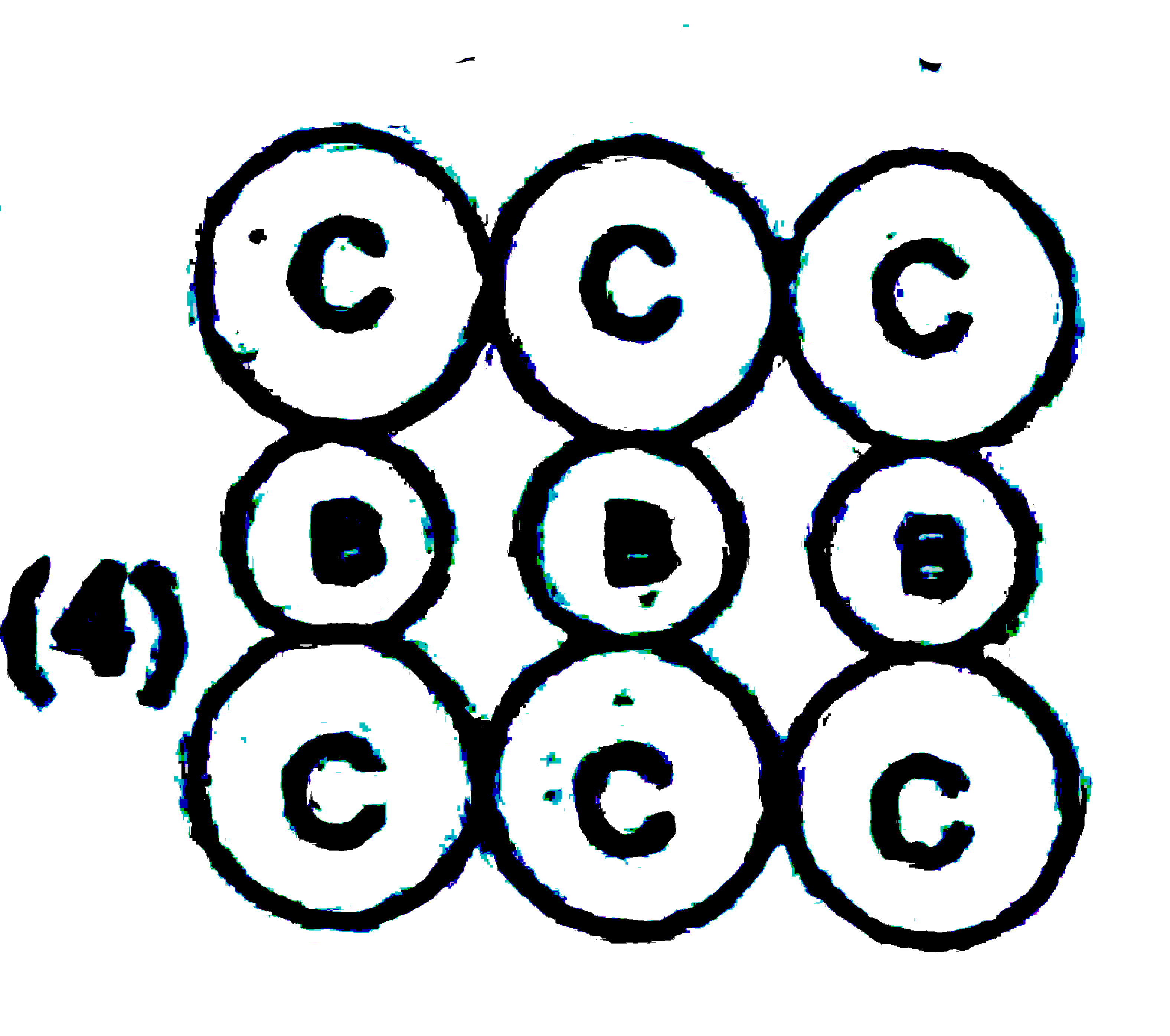
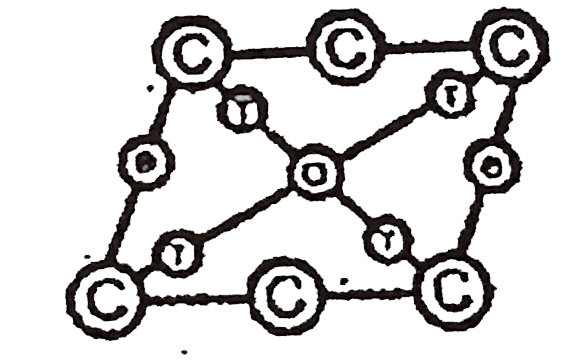
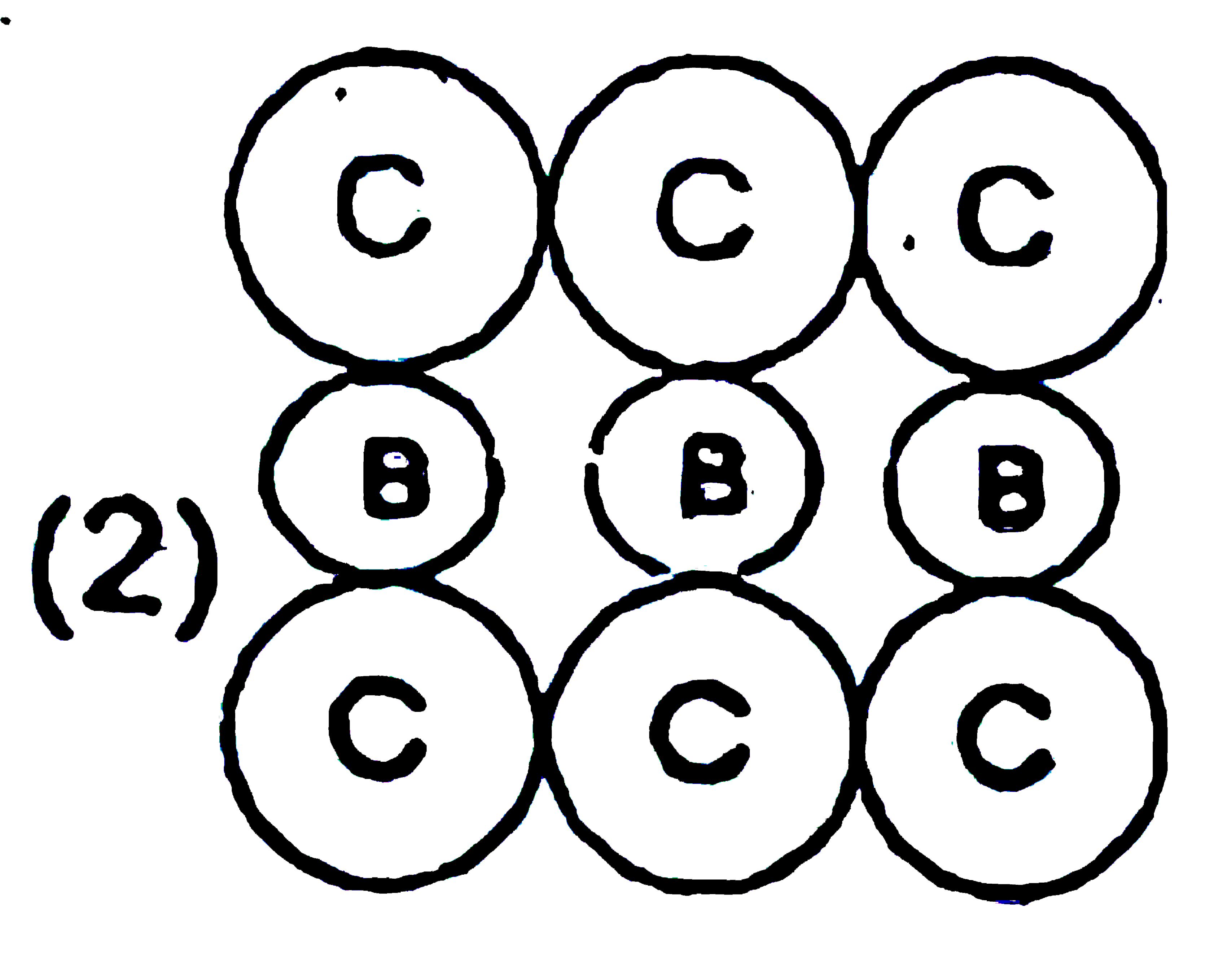A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-SOLID STATE-PHYSICAL CHEMITRY (SOLID STATE)
- Sodium (Na =23) crystallizen in bcc arrangement with the interfaci...
Text Solution
|
- In F.C.C. arrangement of identical spheres, distance between two neare...
Text Solution
|
- In a hypothetical solid C atoms form C C P lattice with A atoms occupy...
Text Solution
|
- Which of the following is incorrect
Text Solution
|
- In the fluorite structure if the radius ratio is (sqrt(3/(2))-1) how m...
Text Solution
|
- The density of KBr is "2.75 g/cm"^3 and edge length of unit cell is 65...
Text Solution
|
- A hypothetical ionic compound AB ( mol. Wt. =240 g//mol e) , having co...
Text Solution
|
- An fcc lattice has a lattice parameter a = 400 pm. Calculater the mola...
Text Solution
|
- A metal crystallises into two cubic phases , face centred cubic (fcc) ...
Text Solution
|
- The arrangement ABC ABC….is referred to as
Text Solution
|
- The pH of a solution of NH4Cl is 5.86. calculate the molar concentrati...
Text Solution
|
- KCl has NaCl type face centred cubic crystal structure and CsF has CsC...
Text Solution
|
- In an ionic solid r((+))=1.6A and r((-))=1.864A. Use the radius ratio...
Text Solution
|
- A crystal is made up of particals X, Y, and Z. X froms fcc packing. Y ...
Text Solution
|
- In hexagonal close packing of shpere in three dimesions, which of the ...
Text Solution
|
- A compound representing alloy of gold and copper crystallises in a cub...
Text Solution
|
- The following diagram shows the arrangement of lattice points with a =...
Text Solution
|
- The silver perchlorate benzene complex, AgClO(4).C(6)H(6) is orthorhom...
Text Solution
|
- CsBr has bcc stucture with edge length 4.3 A .The shortest interionic ...
Text Solution
|
- A solid has a bcc structure. If the distance of closest approach betw...
Text Solution
|