A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-SOLUTION AND COLLIGATIVE PROPERTIES-PHYSICAL CHEMITRY (SOLUTION & COLLIGATIVE PROPERTIES)
- Which of the following statement(s) is/are correct, if intermolecular ...
Text Solution
|
- An aqueous solution of 0.1 molal concentration of sucrose should have ...
Text Solution
|
- When 250 mg of eugenol is added to 100 g of camphor (k(f)=39.7" molali...
Text Solution
|
- The freezing point of a 5g CH(3)COOH (aq) per 100g water is 01.576^(@...
Text Solution
|
- 6.0 g of urea (molecular mass = 60) was dissolved in 9.9 moles of wate...
Text Solution
|
- Calcuate the mass of non-volatile solute having molecoles mass 40, wh...
Text Solution
|
- At 25^(@)C, the vapour pressure of pure liquid A (mol. Mas = 40) is 10...
Text Solution
|
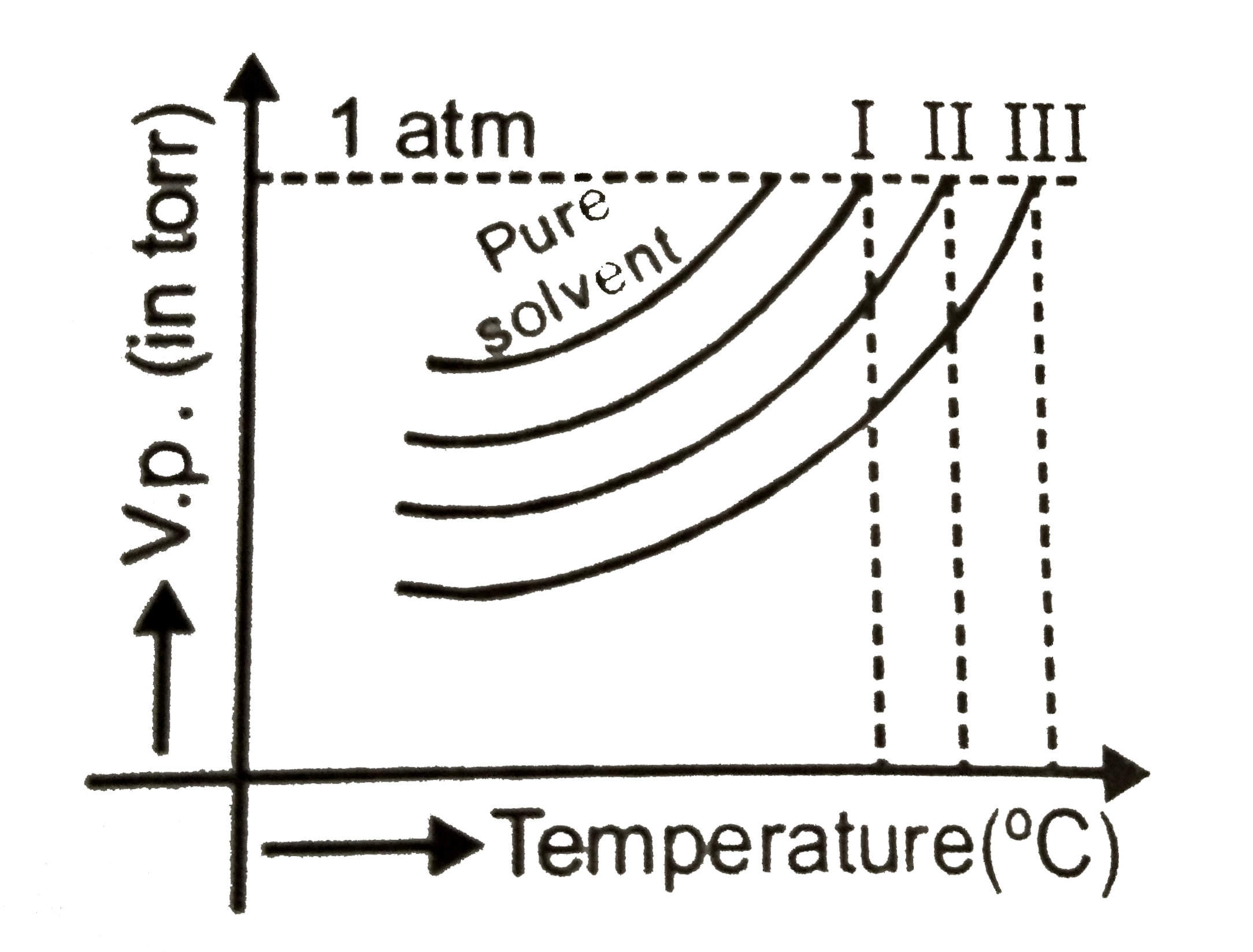
- The vapour pressure curves of the same solute in the same solvent are...
Text Solution
|
- Total vapour pressure of mixture of 1 mole of volaile components A (P...
Text Solution
|
- Water and chlorobenzene are immiscible liquids. Their mixture boils at...
Text Solution
|
- The degree of an electrolyte is a and itsvan't Hoff factor is i. The n...
Text Solution
|
- A complex is represented as CoCl(3)xNH(3). Its 0.1 molal solution inwa...
Text Solution
|
- The freezing point of equimolal solution will be highest for :
Text Solution
|
- A 5% solution of cane sugar (M.W.=342) is isotonic with 1 % solution o...
Text Solution
|
- What is the correct sequence of osmotic pressure of 0.01 M aq. Solutio...
Text Solution
|
- X(3)Y(2)(i=5) when reacted with A(2)B(3)(i=5 in aqueous solution gives...
Text Solution
|
- Which of the following curves represents the Henry' s law?
Text Solution
|
- At 300 K, 40 mL of O(3)(g) dissolves in 100g of water at 1.0atm. What ...
Text Solution
|
- 106.2g 1 molal aqueous solutio of ethylene glycol is cooled to -3.72^(...
Text Solution
|
- The freezing point depression constant for water is -1.86^(@)C m^(-1)....
Text Solution
|