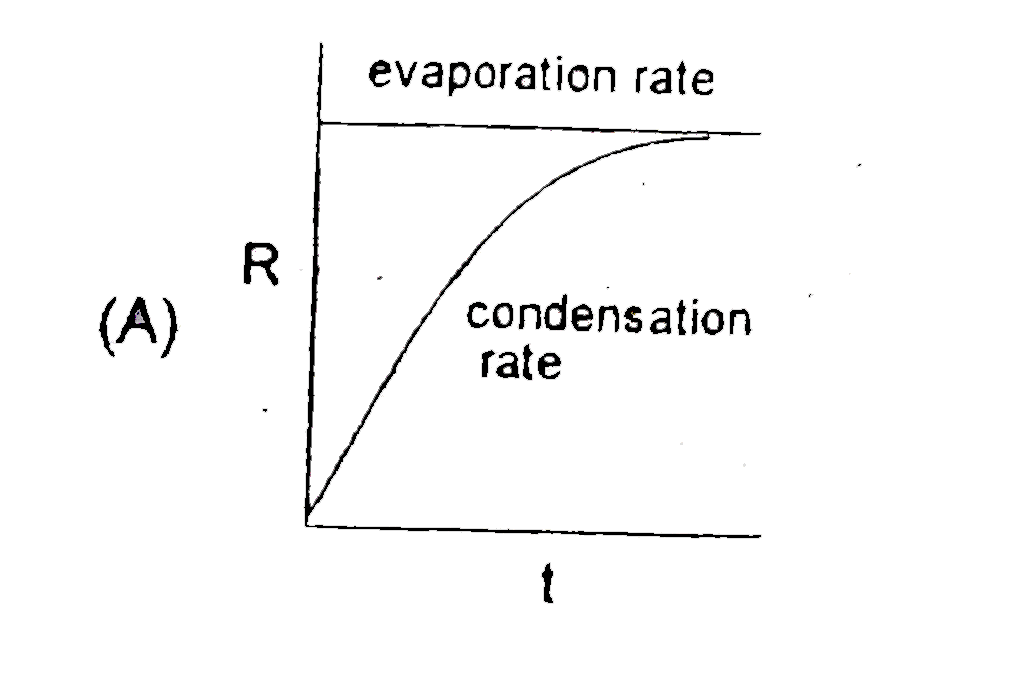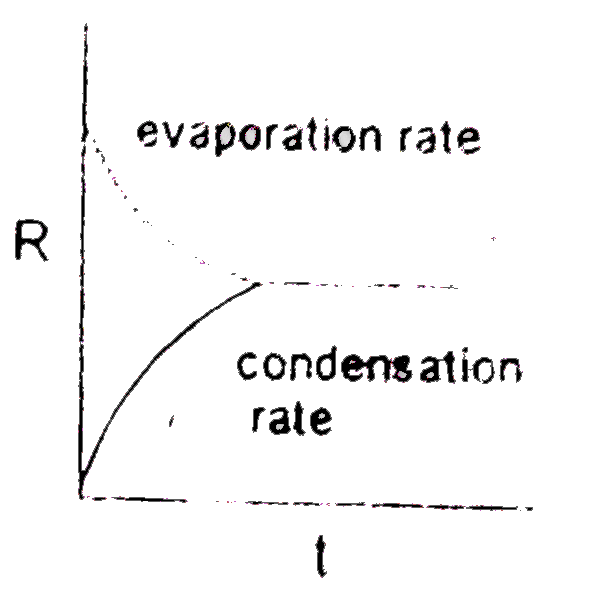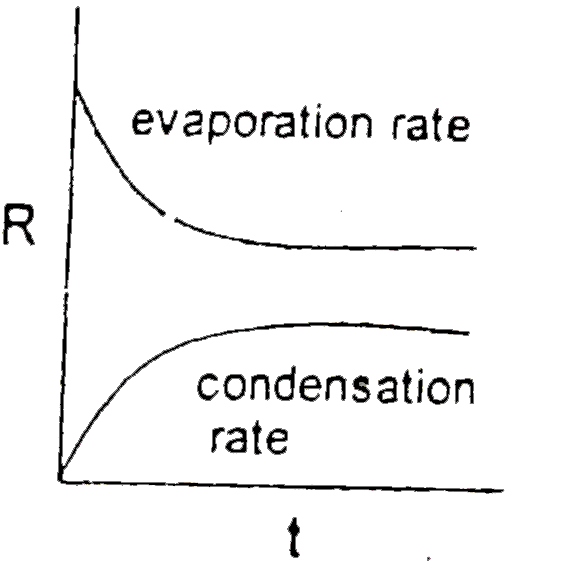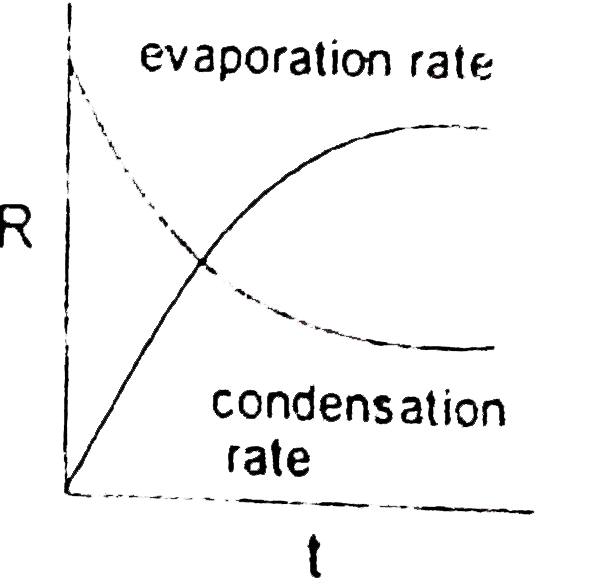A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST PAPERS-Chemistry
- For an ideal solution, if a graph is plotted between (1)/(P(T)) and y(...
Text Solution
|
- For an binary mixture of A and B with p(A)^(0)ltp(B)^(0): x(A)= mole...
Text Solution
|
- Which graph is/are incorrect for a pure liquid evaporating in a clos...
Text Solution
|
- which of the following can form buffer solution
Text Solution
|
- Which of the following is/are exothermic process?
Text Solution
|
- The total vapour pressure of equimolar solution of benzene and toluene...
Text Solution
|
- Which of the following phase diagram is/are not correct for water?
Text Solution
|
- For non ideal solution (positive deviation) which of the following i...
Text Solution
|
- You are given a solution of 50 mL, 0.1 M NaOH, then which of the follo...
Text Solution
|
- Which of the following is/are correctly matched?
Text Solution
|
- Which of the following is/are correct?
Text Solution
|
- Find A,B and C
Text Solution
|
- is this orientation have non-zero dipole moment : Anti conformation of...
Text Solution
|
- An optically active compound A with molecular formula C(8)H(14) underg...
Text Solution
|
- Which of the following rection given secondary alcohol?
Text Solution
|
- Which of the following compounds shows geometrical isomerism ?
Text Solution
|
- Which is/are correct for given structres?
Text Solution
|
- Which set of the following compound(s) give benzene on reaction with P...
Text Solution
|
- Compare the radii of two nuclei with mass number 27 and 125 respective...
Text Solution
|
- Compare the radii of two nuclei with mass number 64 and 1 respectively...
Text Solution
|