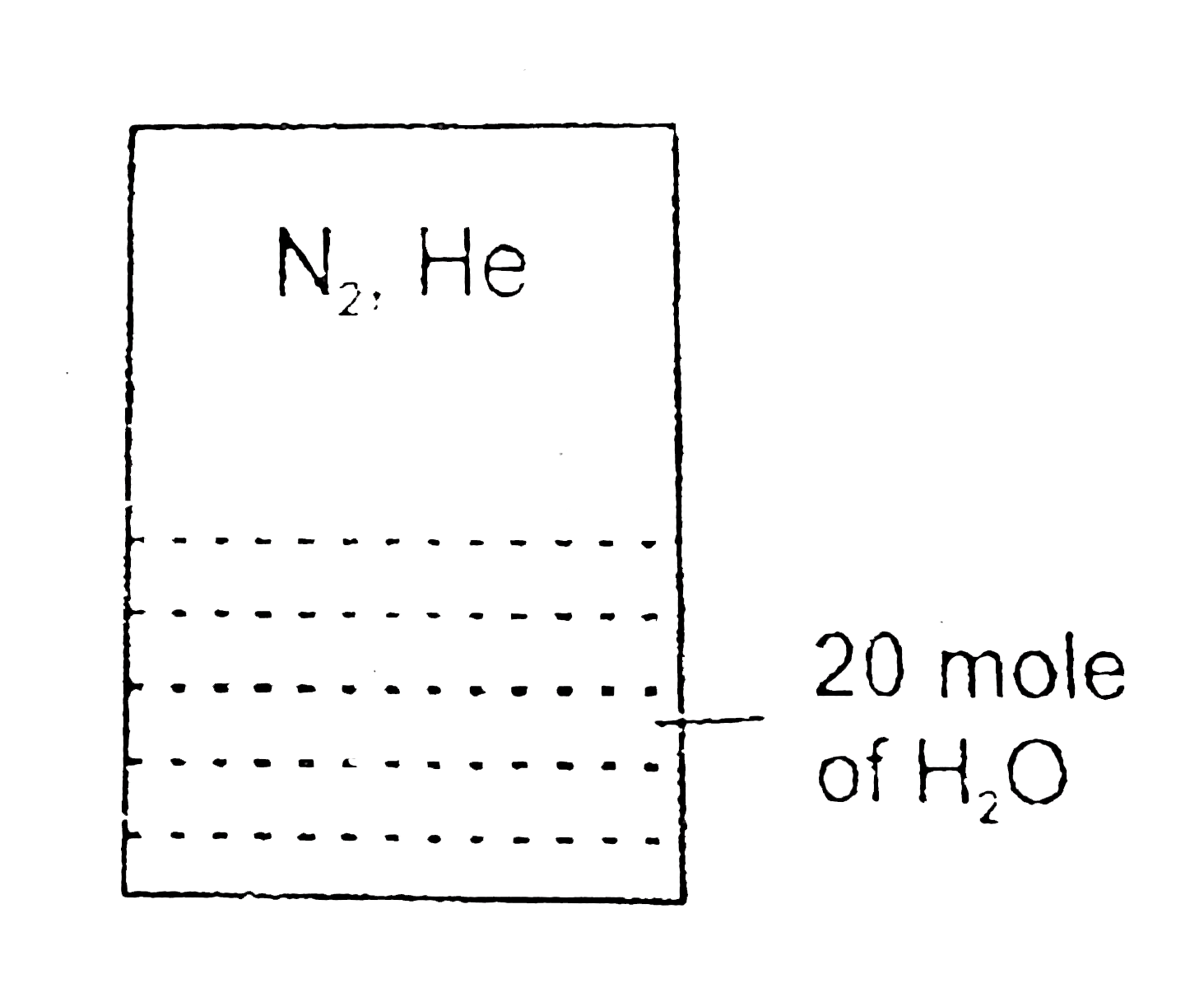Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST PAPERS-Chemistry
- 2 moles of an ideal monoatomic gas undergo a reversible process for wh...
Text Solution
|
- Conductivity of a saturated solution of a sparingly soluble salt AB at...
Text Solution
|
- In the arrangement given below 20 mole of N(2) and 5 mole of He are pr...
Text Solution
|
- An ideal monoatomic gas initially in state 1 with pressure P(1)=20 atm...
Text Solution
|
- How many of the following are bidentate ligan? Oxalate, Glycinate, H...
Text Solution
|
- Which of the following can act as ambidentate ligand? I^(-),NO(2)^(-...
Text Solution
|
- Total number of stereoisomers of given compound are:
Text Solution
|
- Define structure and hybridization of PCl(5)
Text Solution
|
- In following how many structures are chiral?
Text Solution
|
- Calculate the hydrolysis constant of NH4Cl. Determine the degree of hy...
Text Solution
|
- How many total possible products are formed in following reaction: C...
Text Solution
|
- State True or false a chiral carbon consists of 4 different groups A...
Text Solution
|
- A group two element M forms a carbide which is actually a methanide (i...
Text Solution
|
- Which of the following represent(s) bidentate ligand(s)?
Text Solution
|
- Mark the correct statements(s) regarding Azeotropes
Text Solution
|
- A hexa-coordinated complex of formula CoCl(3).6H(2)O undergoes 100% io...
Text Solution
|
- the red coloured Wilkinson's catalyst, [RhCl(PPh(3))(3)] is a homogeno...
Text Solution
|
- Which of the following IUPAC names(s) is/are not correctly matched wit...
Text Solution
|
- Energy profile diagram for an exothermic reaction. Aoverset(1)toBovers...
Text Solution
|
- Consider the following compound: The set of compound/s which give at...
Text Solution
|