A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
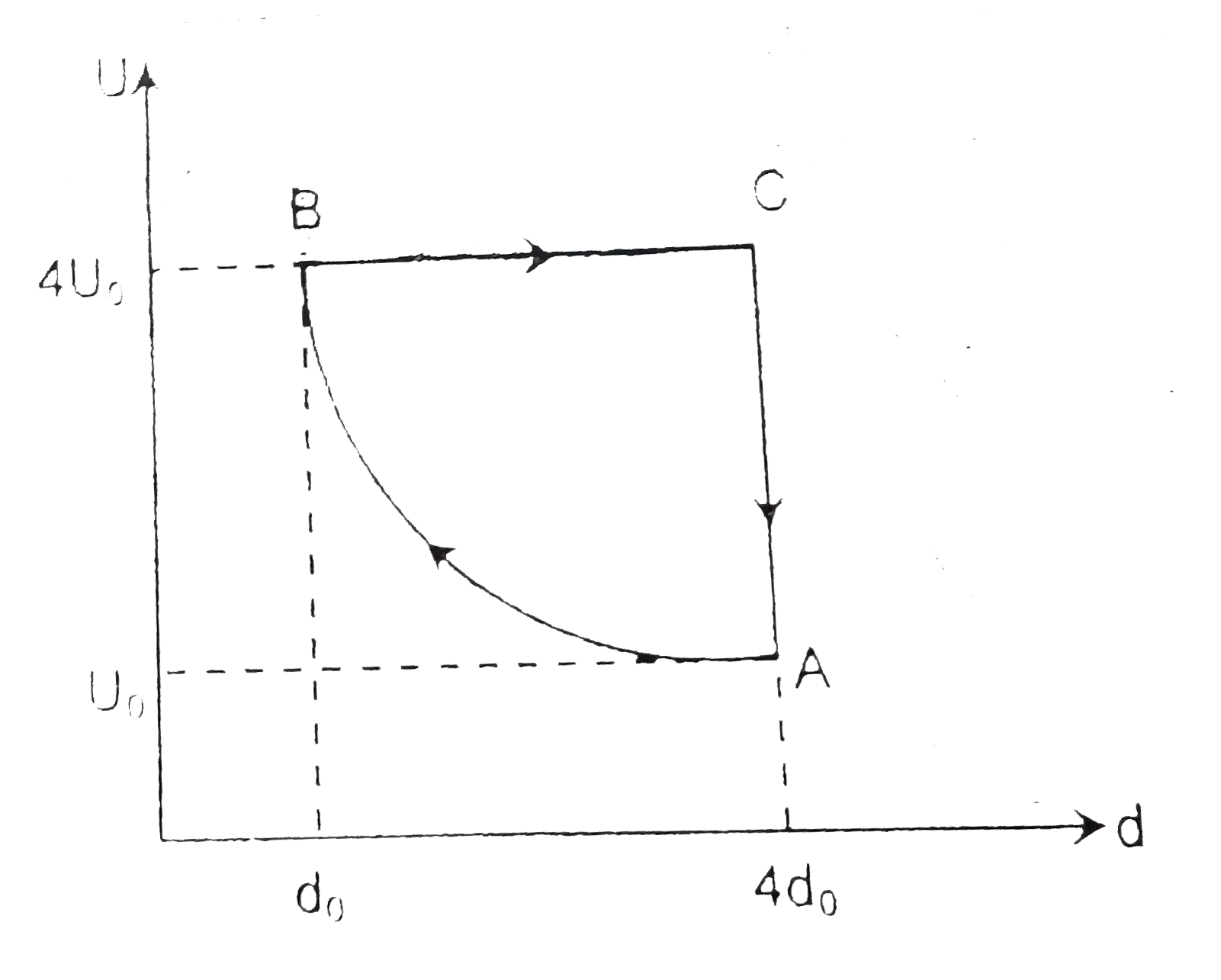
- The variation of internal energy U and density d of one mole of an ide...
Text Solution
|
- Variation of internal energy with density of 1 "mole" of monatomic gas...
Text Solution
|
- One mole of an ideal monatomic gas has initial temperature T(0), is ma...
Text Solution
|
- A thermodynamic process of one mole ideal monoatomic gas is shown in f...
Text Solution
|
- The variation of internal energy U and density d of one mole of an ide...
Text Solution
|
- An ideal monatomic gas undergoes a cyclic process ABCA as shown in the...
Text Solution
|
- One mole of an ideal monatomic gas (intial temperature T(0)) is made t...
Text Solution
|
- Figure shows the variations of the internal energy U With density rho ...
Text Solution
|
- A thermodynamic process of one mole ideal monatomic gas 2.is shown in ...
Text Solution
|