A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An ideal gas can be expended from an initial state to a certain volume...
Text Solution
|
- An ideal gas expands in such a manner that its pressure and volume can...
Text Solution
|
- An ideal gas can be expanded from an initial state to a certain volume...
Text Solution
|
- An ideal gas can be expanded form an initial state to a certain volume...
Text Solution
|
- An ideal gas expands in such a way that PV^2 = constant throughout the...
Text Solution
|
- The pressure P and volume V of a gas are related as PV^(3//2)=K where ...
Text Solution
|
- An ideal gas is taken through a process in which the process equation ...
Text Solution
|
- In a thermodynamic process two moles of a monatomic ideal gas obeys PV...
Text Solution
|
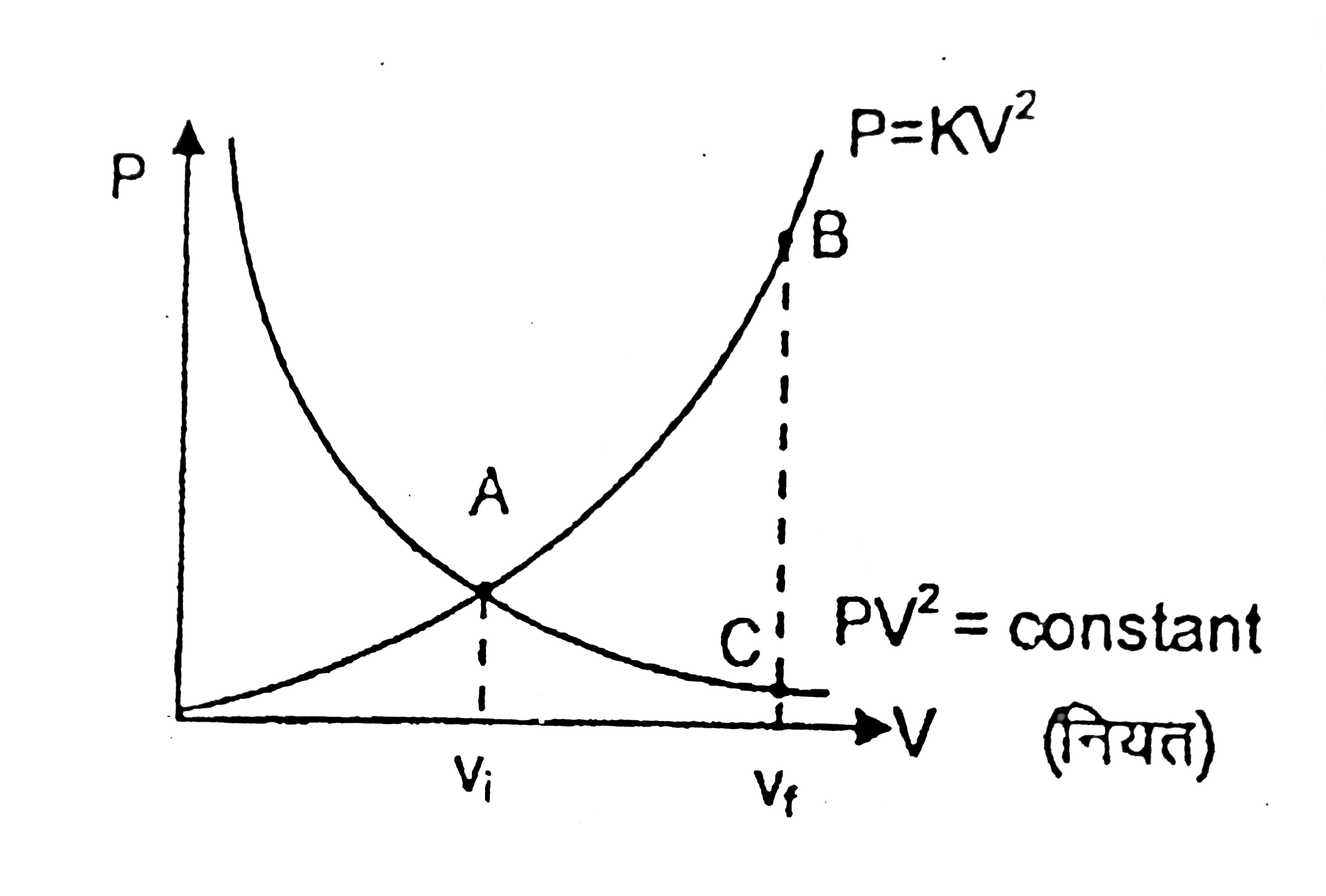
- एक आदर्श गैस इस प्रकार प्रसारित होती है कि इसका दाब व आयतन नियम PV^(2)...
Text Solution
|