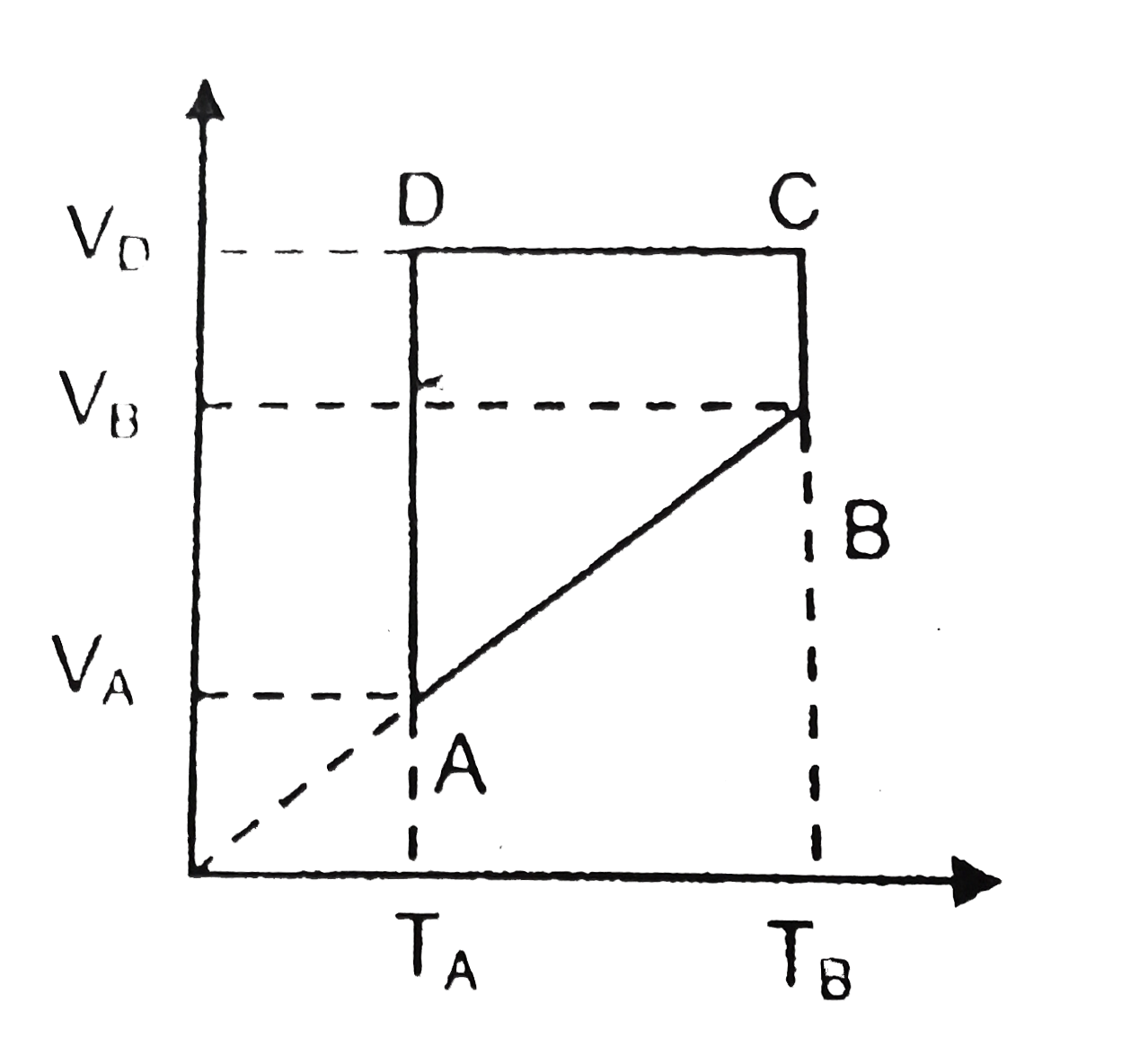
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process starti...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process starti...
Text Solution
|
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|
- A monotomic ideal gas of two metal is taken through a cyclic process s...
Text Solution
|
- A monotomic ideal gas of two metal is taken through a cyclic process s...
Text Solution
|