A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
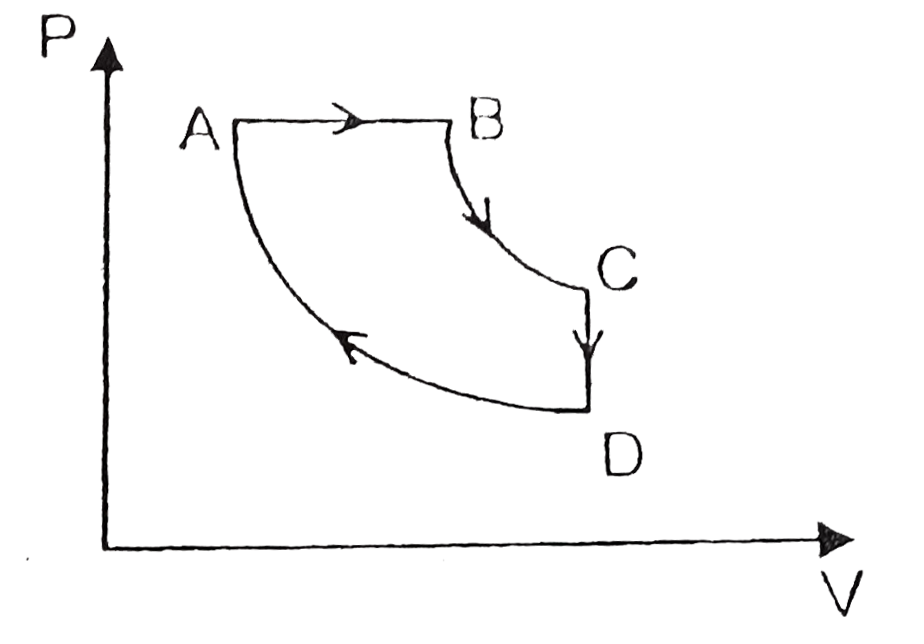
- As shown in figure process AB is isobaric, BC is adiabasic CD is isoch...
Text Solution
|
- n moles of a monoatomic gas undergoes a cyclic process ABCDA as shown....
Text Solution
|
- As shown in figure process AB is isobaric, BC is adiabasic CD is isoch...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- A monoatomic ideal is used as the working substance for the carnot cyc...
Text Solution
|
- One mole of an ideal gas is carried through the reversible cyclic proc...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process as show...
Text Solution
|