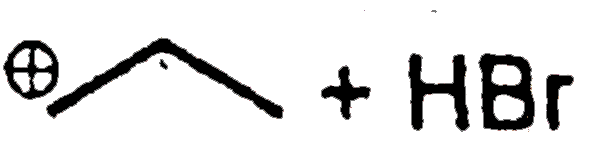
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is a step in the mechanism of the reaction show...
Text Solution
|
- Which of the following is a step in the mechanism of the reaction show...
Text Solution
|
- CH(3)-underset(CH(3)) underset(|) (C)= CH(2) underset("Peroxide") over...
Text Solution
|
- IUPAC name of CH(2)=CH-CH (CH(3)CH(2))underset(Br)underset(|)(C)=CH(...
Text Solution
|
- Give the IUPAC names of the following compounds: (i) C(6)H(5)-CH(2)-...
Text Solution
|
- IUPAC name of CH(2)=CH-CH(CH(3)CH(2))underset(Br)underset(|)C=CH(2) is
Text Solution
|
- CH(3)-CH(2)-CH=CH(2)+HBroverset("ROOR (peroxide)")tounderset("Major")(...
Text Solution
|
- IUPAC name of CH(2)=CH-CH(CH(3)CH(2))underset(Br)underset(|)C=CH(2) is
Text Solution
|
- IUPAC name of CH(2)=CH-CH(CH(3)CH(2))underset(Br)underset(|)C=CH(2) is
Text Solution
|