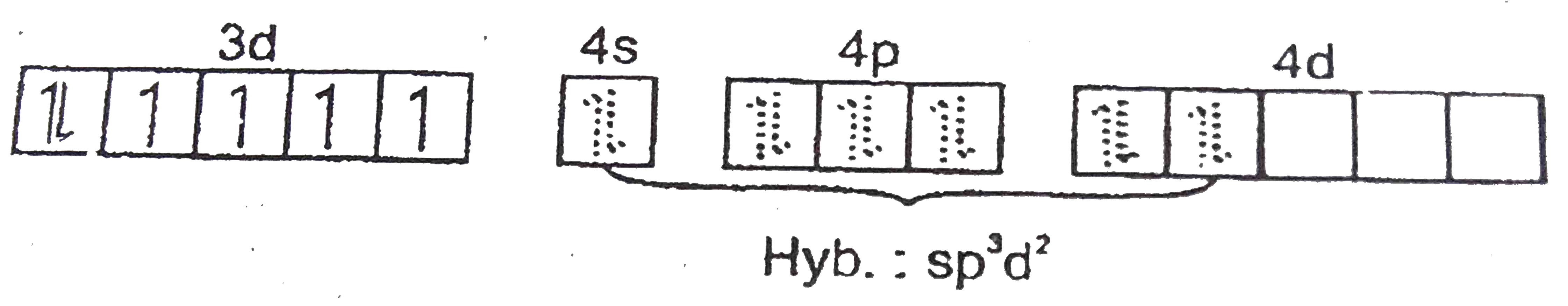
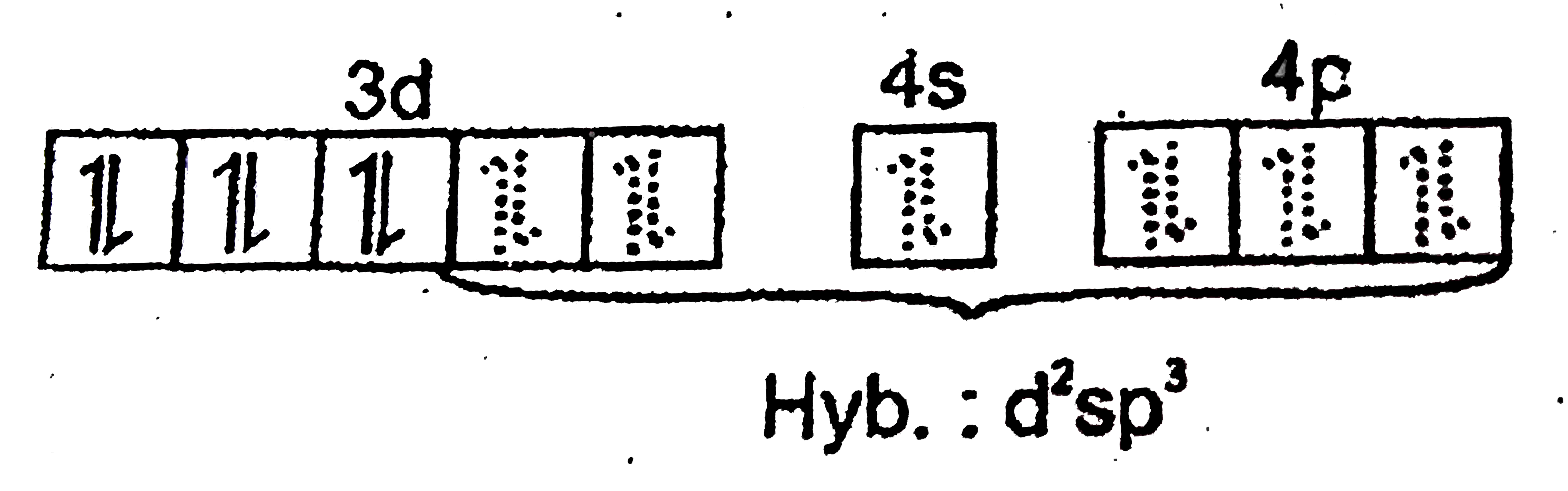
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Hydrocarbon)|7 VideosCOORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise Miscellneous Solved Problem (MSPS)|20 VideosCOORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise Additional Problem for Self Practice (APSP) Part-IV Practice Test -2 (Section-5) (Matching Lift type )|1 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)|15 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-COORDINATION COMPOUNDS-INORGANIC CHEMISTRY (COORDINATION COMPOUNDS)
- The half-life of a radioactive isotope is 2.5 hour. The mass of it tha...
Text Solution
|
- In which of the following pairs both the complex's show optical isomer...
Text Solution
|
- What will be the correct order for the wavelengths of absorption in th...
Text Solution
|
- The half life of a radioactive isotope is 3 hours. If the initial mass...
Text Solution
|
- Given the molecular of the hexacoordinated complexes is : (1)CoCl(3)...
Text Solution
|
- Which of the following pairs of complexes are isomeric with each thei...
Text Solution
|
- The hybridisation of [NiCl(2)(PPh(3))(2)] and [NiCl(2)(PMe(3))(2)] are...
Text Solution
|
- In which of the following pairs both the complexes show geometrical is...
Text Solution
|
- How many total ionization isomers of the complex [CoCl(en)(2)NO(2)]SCN...
Text Solution
|
- The number of electrons in the L-shell of the element with atomic numb...
Text Solution
|
- Which of the following has longest C-O bond length? (Free C-O bond len...
Text Solution
|
- Which of the following orders is incorrect for the properties indicate...
Text Solution
|
- Choose the correct option for the complex [PtCl(2)(en)(2)]^(2+).
Text Solution
|
- How many geometrical isomers and stereoisomers are possible for [Pt(NO...
Text Solution
|
- Dimethyl glyoxime forms a square planar complex with Ni^(2+) . This co...
Text Solution
|
- What is the magnetic moment ( spin only) and hybridisation of the brow...
Text Solution
|
- [Fe(H2O)(6)]^(2+) and [Fe(CN)(6)]^(4-) differ in :
Text Solution
|
- The two compounds pentaamminesulphatocobalt (III) bromide and pentaa...
Text Solution
|
- Select the correct code about complex [Cr(NO(2))(NH(3))(5)][ZnCl(4)] :...
Text Solution
|
- An aqueous solution of titanium bromide shows zero magnetic moment. As...
Text Solution
|