A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
METALLURGY
RESONANCE ENGLISH|Exercise INORGANIC CHEMISTRY(Metallurgy)|42 VideosMETALLURGY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|17 VideosIUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE ENGLISH|Exercise Advanced Level Problems Part-5|2 VideosNITROGEN & OXYGEN FAMILY
RESONANCE ENGLISH|Exercise Part-III Practice Test-2 (JEE (Advanced Pattern))|23 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-METALLURGY-ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)
- In purification of bauxite ore, it is mixed with coke and heated at 18...
Text Solution
|
- If wavelength of photon is 1.1×10 ^(−11) m, h=6.6×10 ^(−34)Jsec, then...
Text Solution
|
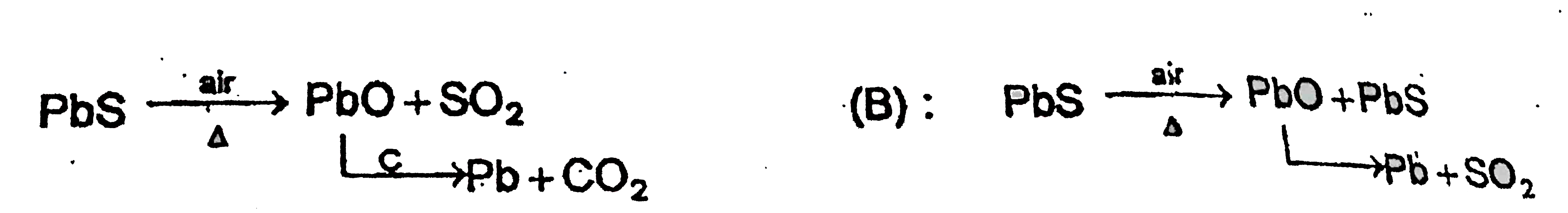
- Identify X and Y in the following reactions . PbS underset("in air")...
Text Solution
|
- If wavelength of photon is 2.2×10 ^(−9) m, h=6.6×10 ^(−34)Jsec, then ...
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- If wavelength of photon is 3×10 ^(−18) m, h=6.6×10 ^(−34)Jsec, then m...
Text Solution
|
- The sulfide ores are generally concentrated by
Text Solution
|
- Which one of the following sulphide ores is concentrated by chemical l...
Text Solution
|
- NaCN is sometimes added in the forth floatation process as depressant ...
Text Solution
|
- Which of the following process is related with the removal of sulphur ...
Text Solution
|
- Consider the following statements : Roasting is carried out to : 1...
Text Solution
|
- Main source of lead is galena (PbS). It is converted to Pb by : S...
Text Solution
|
- The chemical composition of slag formed during the smelting process in...
Text Solution
|
- Pb and Sn are extracted from their chief ore respectively by
Text Solution
|
- Poling process :
Text Solution
|
- In the process of extraction of gold , Roasted gold ore + CN((aq))^...
Text Solution
|
- In electrorefining the impure metal is made
Text Solution
|