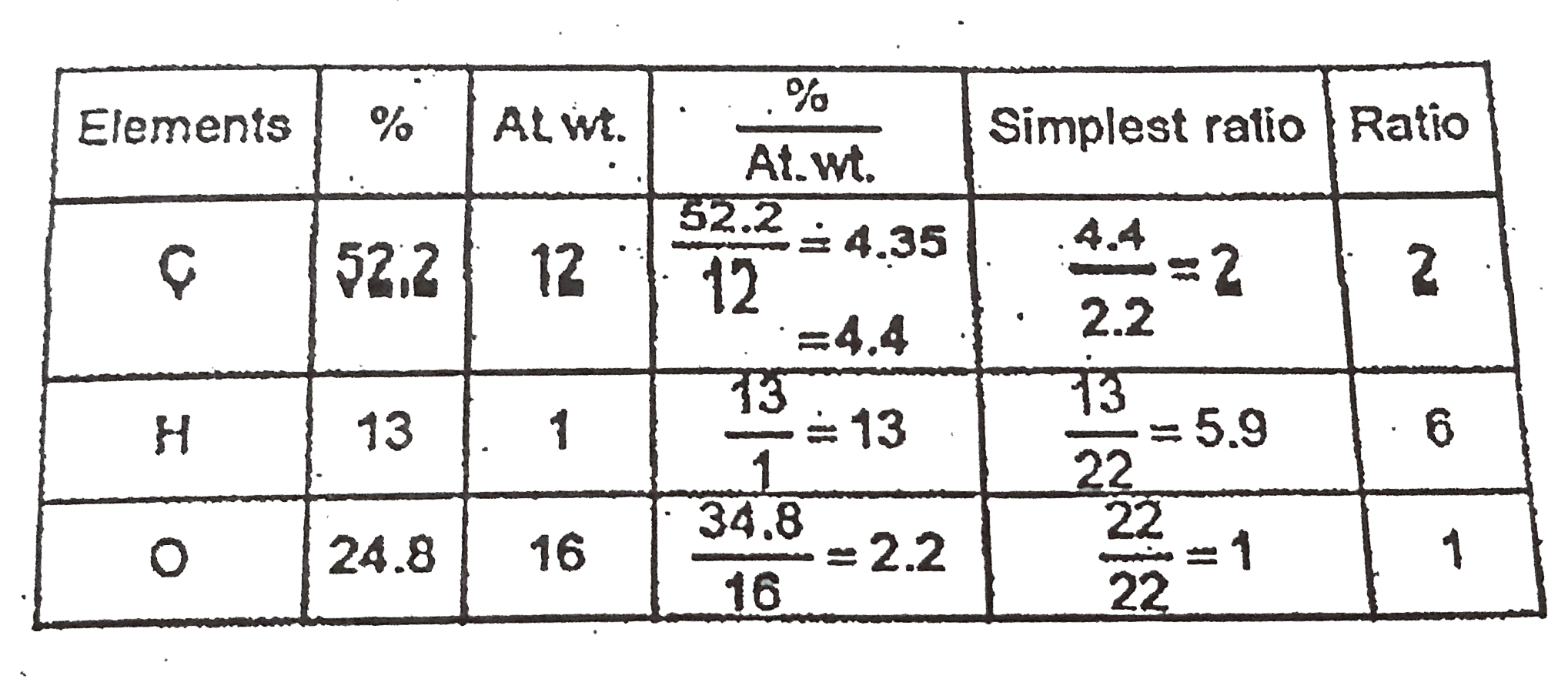
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise Inorganic chemistry (Periodic Table & Periodicity|61 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-3|22 VideosREDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Comprehension Type|3 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-PERIODIC TABLE & PERIODICITY-ORGANIC CHEMISTRY(BASIC CONCEPTS)
- The first ionisation potential of Al is smaller than that of Mg becaus...
Text Solution
|
- In which of the following pairs, the first member has higher first ion...
Text Solution
|
- Which one of the following atoms will have the smallest size ?
Text Solution
|
- The chemical formula of Permanganic acid is -
Text Solution
|
- Which of the following is the correct order of ionisation enthalpy ? ...
Text Solution
|
- The statement that is not correct for periodic classification of eleme...
Text Solution
|
- In the modern periodic table, the block indicates the value of ……………… ...
Text Solution
|
- Element with electronic configuration as [Ar]^(18)3d^(5)4s^(2) is plac...
Text Solution
|
- Which set does not shows correct matching according to Modern periodic...
Text Solution
|
- Atomic radii of fluorine and neon in Angstrom units are respectively g...
Text Solution
|
- Which of the following element has the highest ionisation enregy ?
Text Solution
|
- The set representing the correct order of the first ionisation potenti...
Text Solution
|
- Which of the following relation is correct with respect first (I) and ...
Text Solution
|
- First, second & third ionization energies are 737, 1045 & 7733 KJ//mol...
Text Solution
|
- For which of the following process, the value of electron gain enthalp...
Text Solution
|
- Which is the correct property mentioned -
Text Solution
|
- If ionisation energy of an atom is 10 eV & EA is 6.8eV electronegativi...
Text Solution
|
- Fluorine has the highest electronegativity among the ns^(2) np^(5) gro...
Text Solution
|
- The increasing order of electron affinity values of O,S and Se is
Text Solution
|
- Electron addition will be easier in : -
Text Solution
|