A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST PAPERS-Chemistry
- Identify the option which represents the correct products of the follo...
Text Solution
|
- can be reduced to by the reagents.
Text Solution
|
- Which is (are) correct out of the following:
Text Solution
|
- Identify the correct options related with the physical properties of ...
Text Solution
|
- Choose the incorrect option (s):
Text Solution
|
- In which of the following reactions correct major product has be menti...
Text Solution
|
- Which of the following are correct regarding the given reaction?
Text Solution
|
- The K(a) of monobasic acid P, Q and R are 10^(-6),10^(-8) and 10^(-10)...
Text Solution
|
- The K(a) of monobasic acid P, Q and R are 10^(-6),10^(-8) and 10^(-10)...
Text Solution
|
- At infinite dilution, the equivalent conductances of CH(3)COOHa, HCl a...
Text Solution
|
- At infinite dilution, the equivalent conductances of CH(3)COOHa, HCl a...
Text Solution
|
- Synthesis of propellane takes placed by the following route. Q. Prod...
Text Solution
|
- Synthesis of propellane takes placed by the following route. Q. The ...
Text Solution
|
- How many electron in Ca (Z =20) have n = 2, l = 0 ?
Text Solution
|
- What is pH of pure water whose is degree of dissociation 18xx10^(-9)?
Text Solution
|
- For a cell reaction DeltaG^(@)=-1930(kJ)/(mol) and the cell e.m.f (E^(...
Text Solution
|
- How many of the following pairs are conjugate acid/base pairs? HCl,C...
Text Solution
|
- What is sum of oxidation number and coordination number of Al in Cryol...
Text Solution
|
- What is atomicity of gaseous compound formed in the Mond's process whe...
Text Solution
|
- How many monocarboxylic acids (indluding stereo) would give Methylcycl...
Text Solution
|
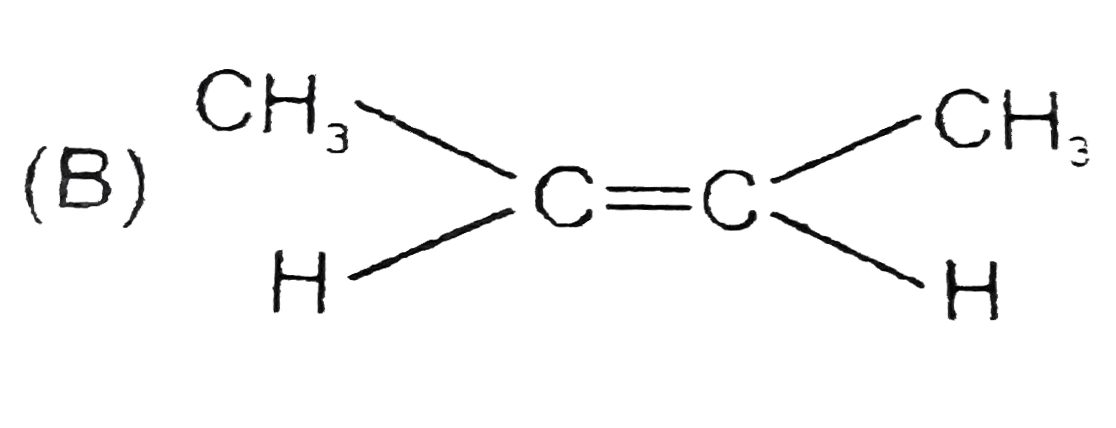 `ltH_(3)C-C-=C-CH_(3)` (rate of catalytic hydrogenation)
`ltH_(3)C-C-=C-CH_(3)` (rate of catalytic hydrogenation)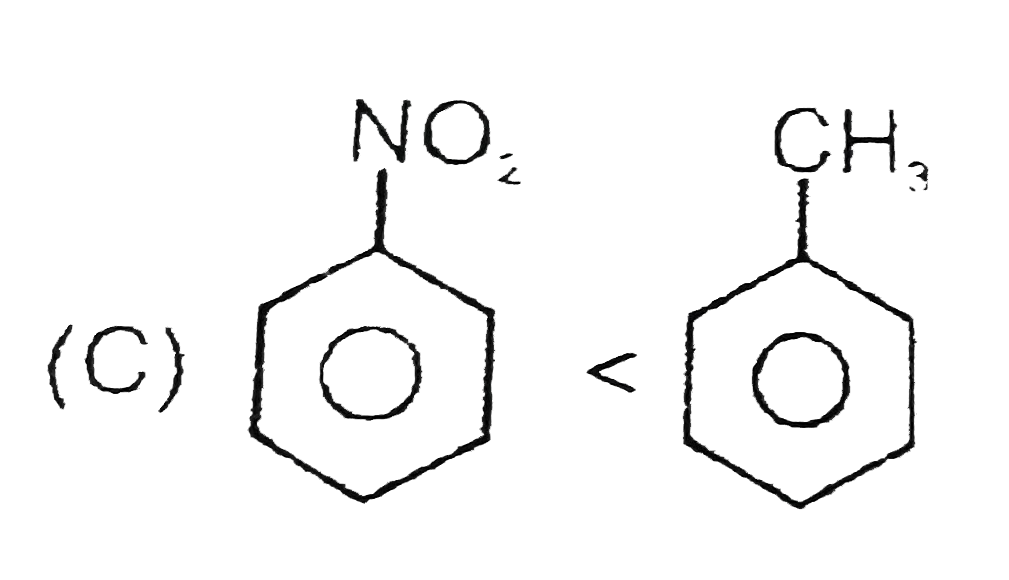 (rate of electrphilic substitution reaction)
(rate of electrphilic substitution reaction)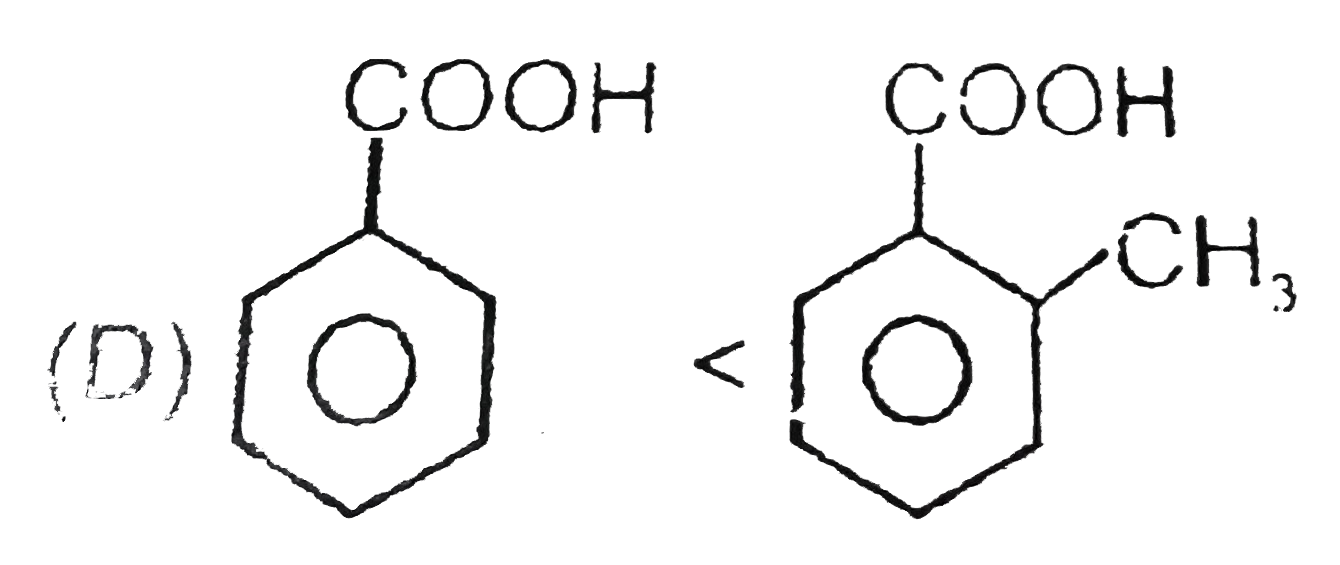 (order of acidic strength)
(order of acidic strength)